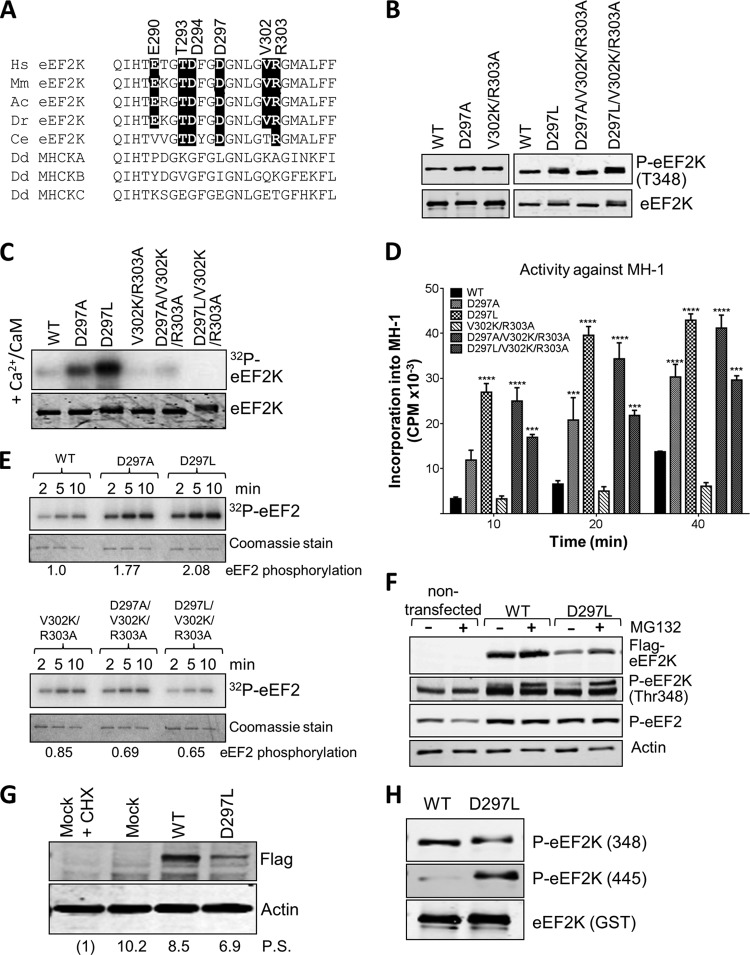
FIG 6.
Mutations at residues in the N/D loop region of eEF2K alter its substrate specificity. (A) Alignment of N/D loop regions from eEF2K from different species against MHCK isoforms, with conserved residues of interest indicated by black boxes. Abbreviations for species are given in the legend to Fig. 2. (B) Recombinant GST-eEF2K and selected mutants were analyzed by SDS-PAGE and Western blotting to determine the level of autophosphorylation using phospho-Thr348 antibody. (C) Recombinant GST-eEF2K or selected point mutants were incubated with alkaline phosphatase (PPase) for 20 min at 30°C before incubation with Ca2+/CaM, as indicated with ATP or [γ-32P]ATP for 10 min at 30°C. Samples were analyzed by SDS-PAGE and phosphorimaging or Western blotting using the indicated antibodies. (D) Activities of selected point mutants against the MH-1 peptide. All assays were performed within the linear range of the assay. Data are means ± SEM (n = 3). ***, P < 0.001; ****, P < 0.0001. (E) Activities of selected point mutants of eEF2K against EF2 were determined. All assays were performed within the linear range of the assay. Samples were analyzed by SDS-PAGE and phosphorimaging. The labeling of eEF2 at the 5-min time point was quantified (values below each set of assays; normalized to labeling of eEF2 by WT eEF2K). (F) Where indicated, HEK293 cells were transfected with vectors encoding WT eEF2K or the D297L mutant. In some cases, cells were treated with MG132 (10 μM, 16 h). Forty hours after transfection, cell lysates were prepared and analyzed by Western blotting using the indicated antibodies. (G) Results of an experiment similar to that shown in panel F, except that protein synthesis was assessed using the SUnSET method. In one case, cells were treated with cycloheximide (CHX) to inhibit protein synthesis (in order to provide a baseline for assessing the true rate of protein synthesis). Mock indicates cells were treated in a similar way as when transfected but no DNA was added. Detection of new protein was achieved using antipuromycin antibodies; the membrane was reprobed with an anti-FLAG antibody. Numbers below each lane indicate relative levels of puromycin incorporation (protein synthesis [P.S.]), determined after subtracting the signal for CHX-treated cells. (H) Recombinant WT GST-eEF2K and the D297L mutant were analyzed by SDS-PAGE and Western blotting using phospho-Thr348 or phospho-Thr445 antibodies. Total amounts were assessed using anti-GST antibody.