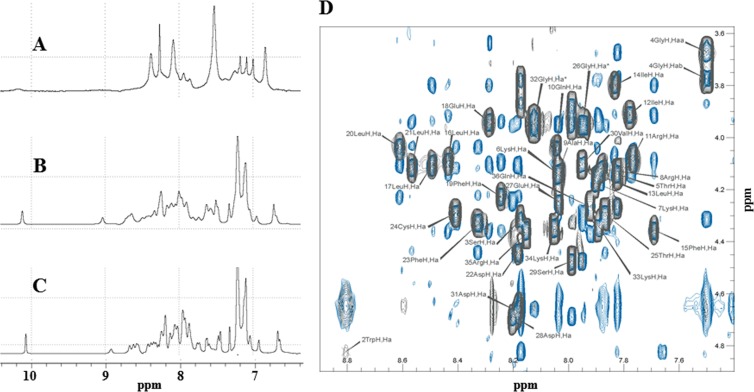
FIG 2.
Comparison of the 1D 1H NMR spectra of the agnoprotein peptide determined under different conditions. The spectra were acquired on an Avance Bruker spectrometer operating at 600.13 MHz and equipped with a cryoprobe. Experiments were carried out to assess the ability of various conditions to increase the solubility of the agnoprotein peptide and to prevent its aggregation. (A) In pure water at pH 3.0, the peptide appears to be aggregated. (B) In the presence of 30% (vol/vol) TFE, pH 3.0, at 293 K, the peptide appears to be structured with a large spreading of the chemical shifts, but the line width remains relatively broad. (C) The assay was performed under the same conditions used for the assay whose results are presented in B but at a temperature of 313 K, which allows spectra with a narrower line width to be obtained. All experiments were conducted using a 0.5 mM concentration of the agnoprotein peptide. (D) A representative NOESY spectrum of the agnoprotein peptide. The spectrum was recorded with a mixing time of 200 ms in the presence of 30% (vol/vol) TFE at pH 3.0 and a temperature of 313 K. Only the region of the spectrum showing the interresidual correlations between the α proton (i) and the amide proton (i + 1) is shown. The intraresidual correlations for each residue (i) are labeled.