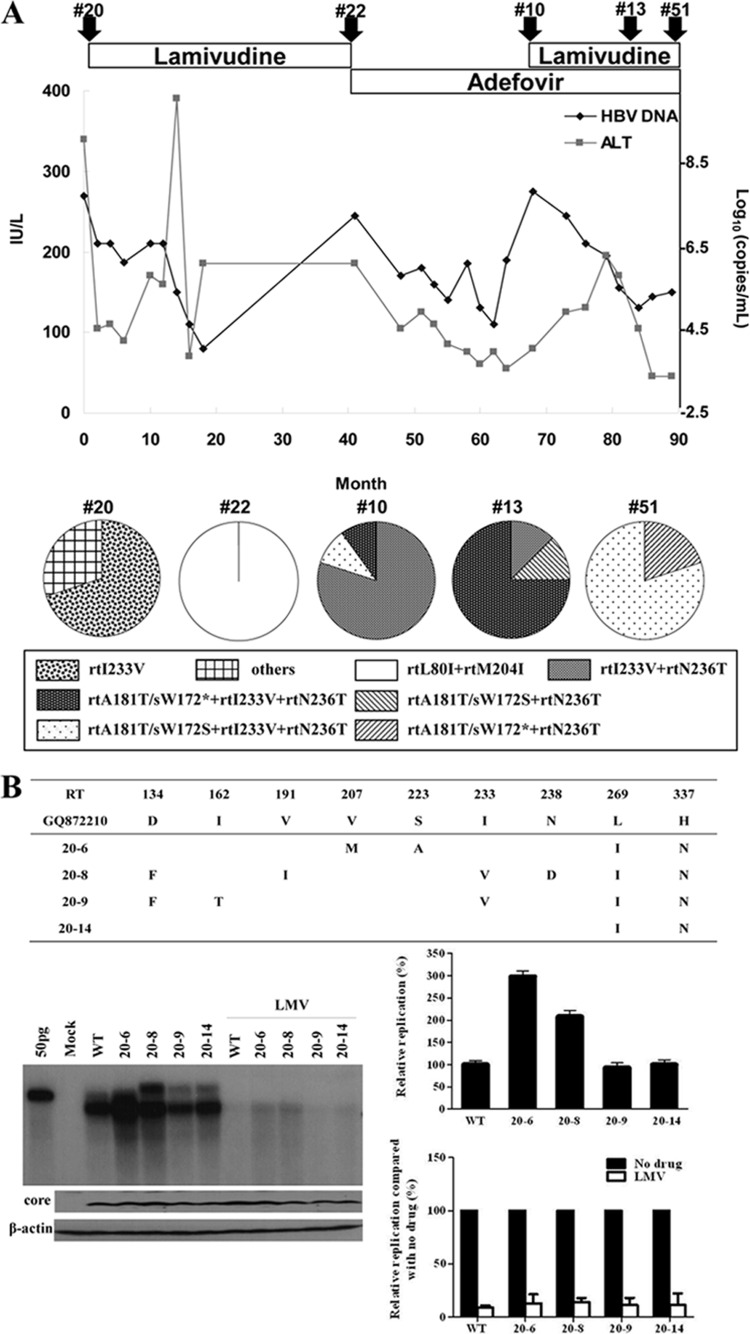
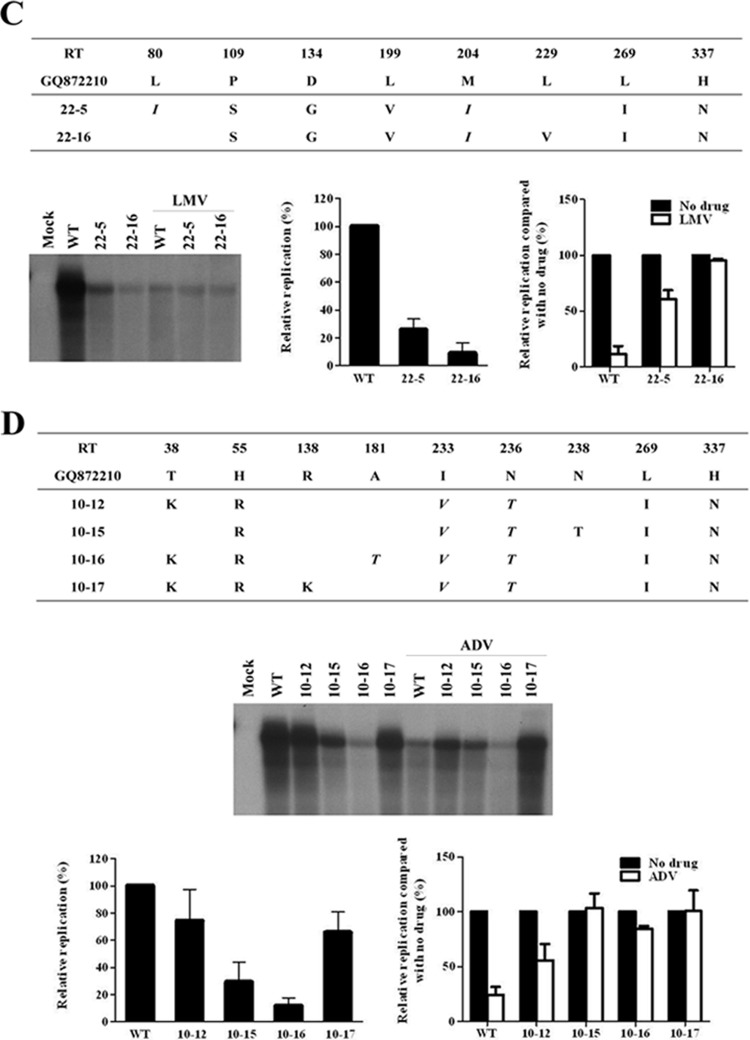
FIG 1.
Clinical characteristics and in vitro susceptibility assay of RT mutants isolated at the time of treatment failure. (A) Clinical course during chronic hepatitis B infection in a patient. Arrowheads indicate the time points of serum sampling used in the present study. Clone series denoted by “#” were selected for further study. Analysis of coexisting HBV quasispecies at the time of viral breakthrough is summarized. (B to D) The HBV RT genes from each serum sample were converted into HBV1.2mer replicons, and the substitutions were compared to the WT. Cloned HBV DNAs were transfected into Huh7 cells, and cells were treated for 4 days with or without 20 μM LMV or ADV. The intracellular HBV DNA was analyzed by Southern blotting. The relative replication ability and drug susceptibility compared to the WT were quantified by using a PhosphorImager. Aliquots of same cell lysates were analyzed by Southern blotting to detect HBV core and actin proteins to verify the transfection yield and loading amount.