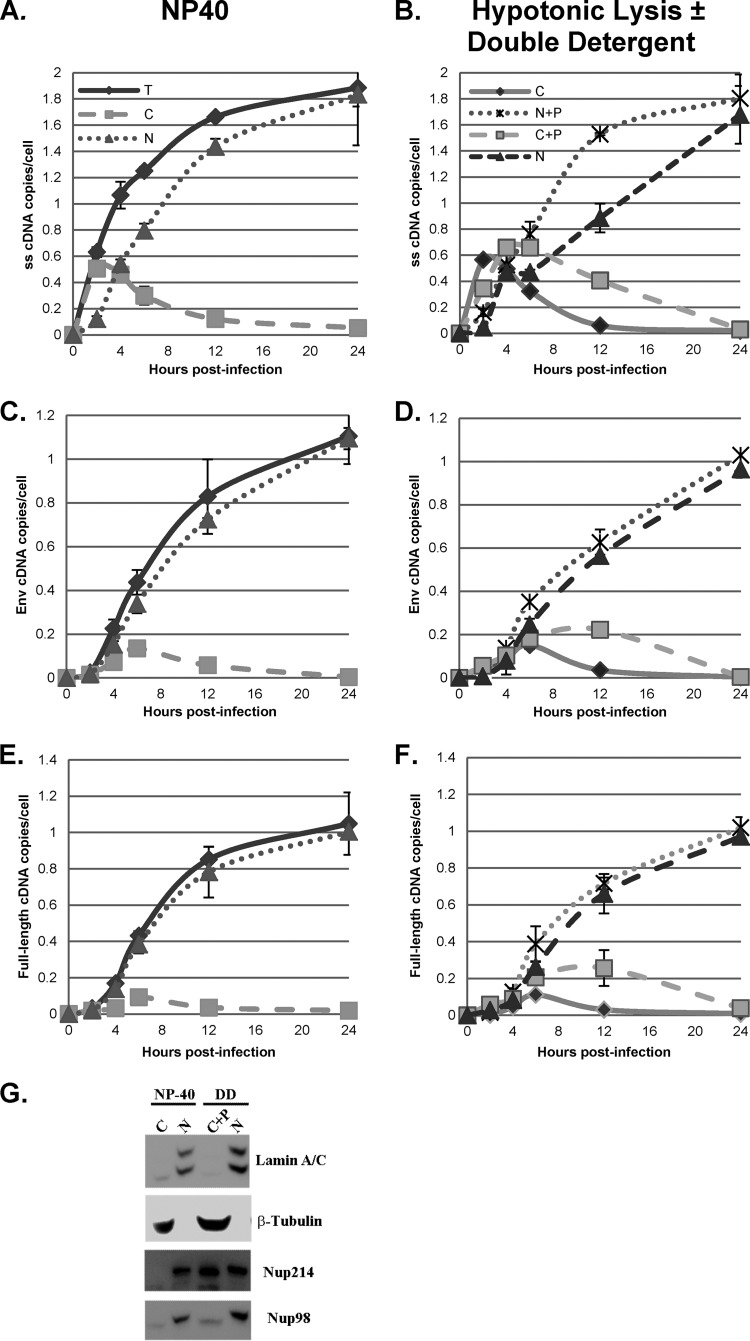
FIG 8.
Comparison of fractionation protocols. HeLa cells were infected with a DNase-treated HIV-1 vector (HR-E). Zero to 24 h later, the cells were lysed to prepare whole-cell (total [T]) fractions or fractionated into nuclei (N) and cytoplasm (C) utilizing the NP-40 protocol (NP-40) (A, C, and E). DNA was isolated for qPCR and analyzed with primers specific for strong-stop (A), env (C), and full-length (E) DNA (Fig. 3). The strong-stop, env, and full-length DNA copy numbers were normalized to the number of copies of either the β-globin DNA (for total and nuclear fractions) or mitochondrial DNA (for the cytoplasmic fraction) standards. Error bars denote the standard deviations from the means. The data represent those from two independent time course experiments processed in triplicate. Similarly infected cells were fractionated by hypotonic swelling and Dounce homogenization and separated into cytoplasmic (C) and nuclear plus perinuclear (N+P) fractions (B, D, and F). In parallel samples, the N+P fractions were treated with double detergent, and the supernatants were combined with the cytoplasmic fractions to give cytoplasmic plus perinuclear (C+P) and nuclear (N) fractions (results are also shown in panels B, D, and F). qPCR for strong-stop, env, and full-length DNA was performed. (G) Proteins from the nuclear and cytoplasmic fractions obtained by NP-40 lysis and from the N and C+P fractions obtained by fractionation with hypotonic lysis and double-detergent (DD) washing of nuclei were analyzed by SDS-PAGE and Western blotting for lamin A/C, β-tubulin, Nup214, and Nup98. Fractions from equal numbers of cells were analyzed.