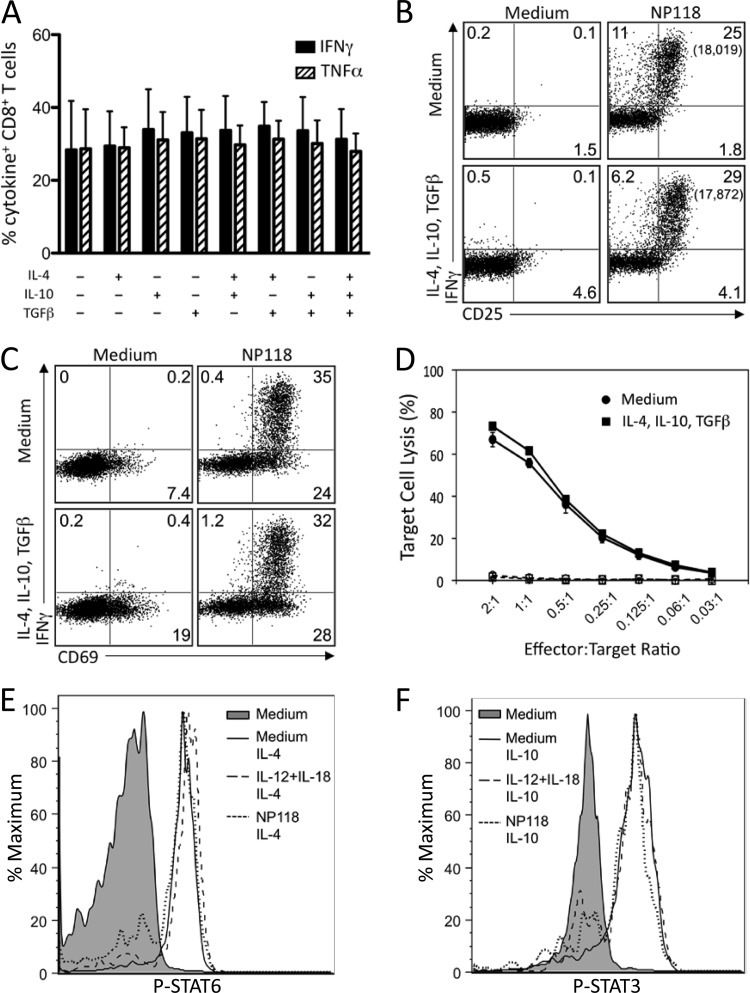
FIG 5.
Anti-inflammatory cytokines do not inhibit peptide-specific CD8+ T cell responses. Splenocytes from BALB/c mice at 8 days post-LCMV infection were treated directly ex vivo with the indicated combinations of IL-4, IL-10, and TGF-β (10 ng/ml each) for 2 h, followed by stimulation with NP118 peptide (1 × 10−7 M) for 6 h. (A) Peptide-specific IFN-γ and TNF-α production by virus-specific CD8+ T cells. Numbers represent the percentage of NP118 tetramer+ CD8+ T cells producing IFN-γ or TNF-α and are the average ± SD from 4 mice. (B and C) Surface marker upregulation in response to peptide stimulation with or without pretreatment with anti-inflammatory cytokines. Numbers in each quadrant represent the percentage of CD8+ T cells expressing IFN-γ and/or CD25 (B) or CD69 (C), while numbers in parentheses represent the MFI of IFN-γ+ T cells. Data are representative of 4 mice from 2 independent experiments. (D) At 8 days following infection with LCMV, T cells were incubated in medium alone or medium containing IL-4, IL-10, and TGF-β for 2 h prior to incubation with NP118-coated (solid lines) or uncoated (dashed lines) target cells for 5 h. Results are representative of 2 independent experiments. (E) At 8 days post-LCMV infection, splenocytes were stimulated with IL-12 plus IL-18, IL-12 plus TNF-α, or IL-12 plus IL-15 (10 ng/ml each) or NP118 peptide directly ex vivo. After 2 h, IL-4 (E) or IL-10 (F) was added for 20 min, cells were immediately fixed and permeabilized, and phosphorylation of STAT3 (E) and STAT6 (F) was assessed. Results are representative of 4 mice and 2 independent experiments.