Abstract
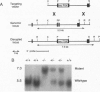
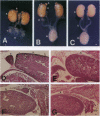
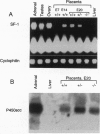
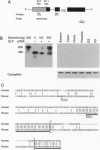
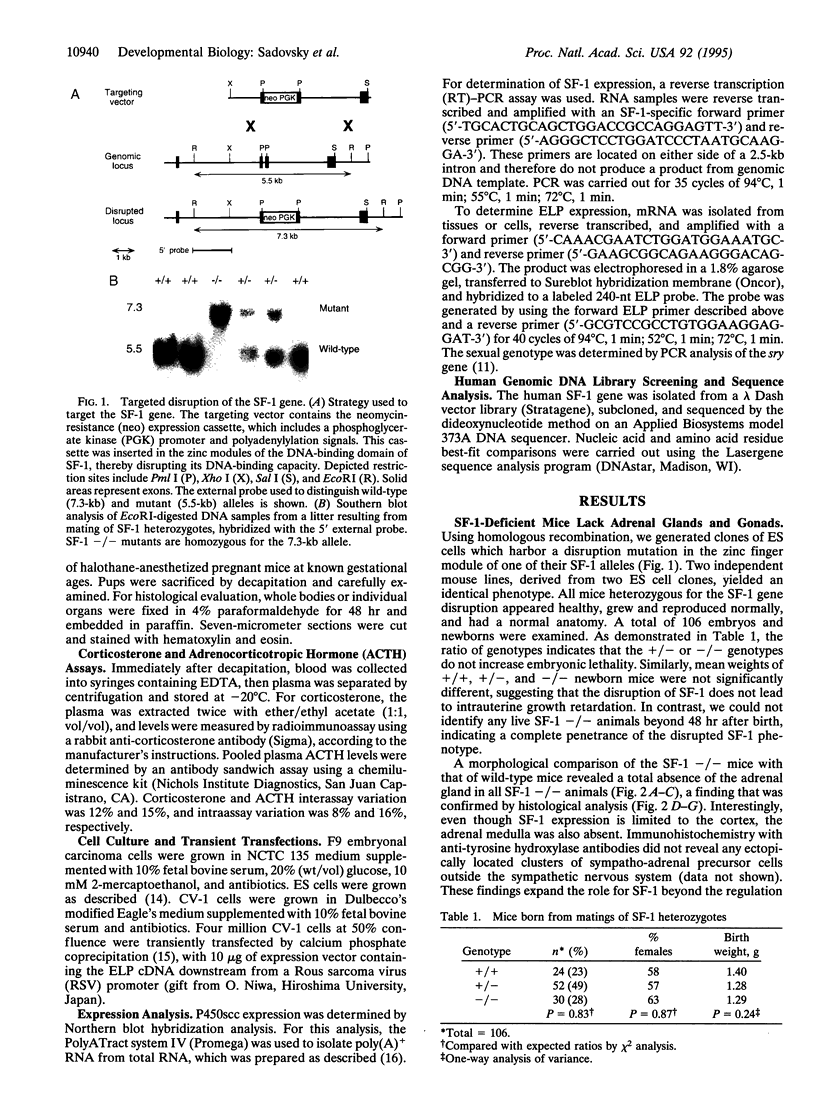
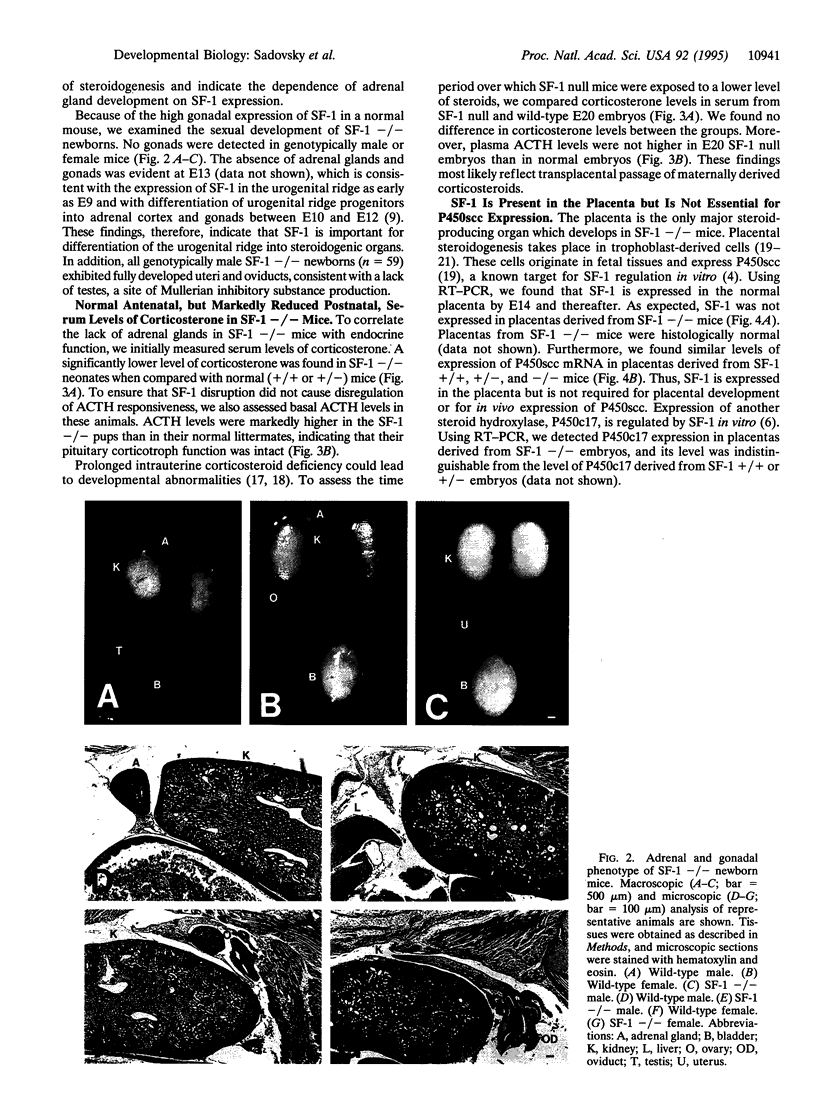
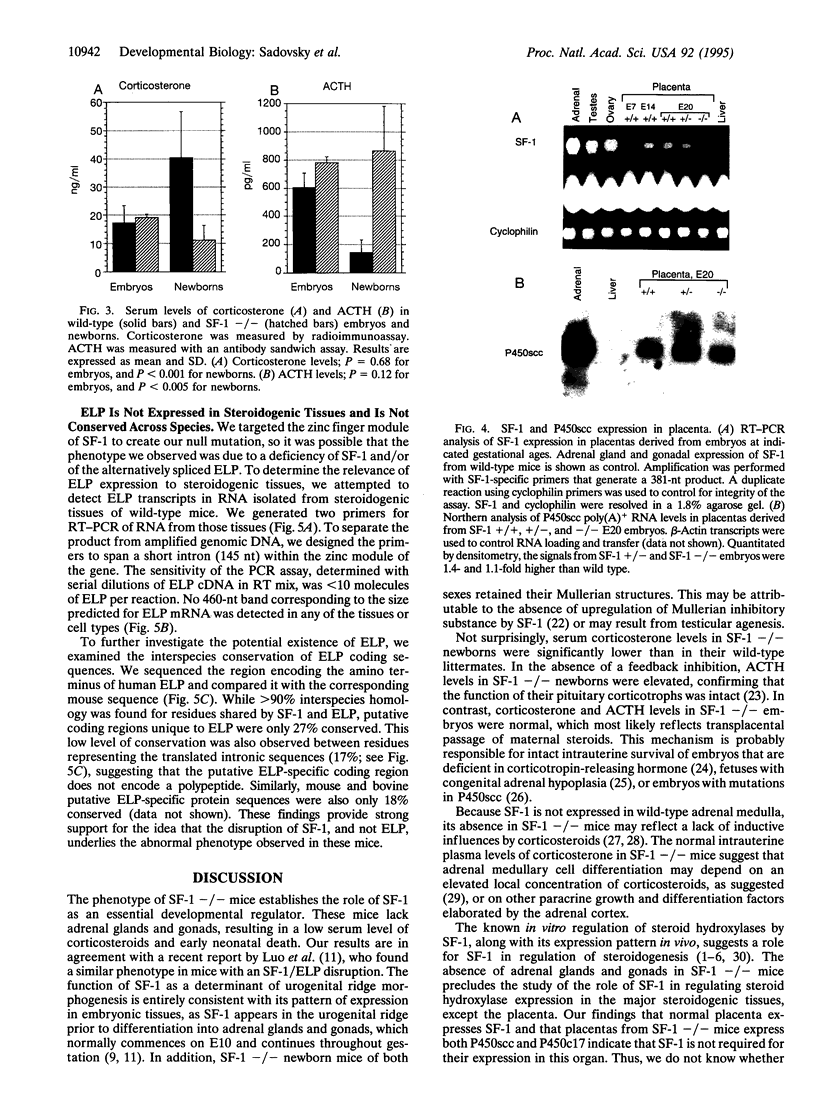
The orphan nuclear receptor steroidogenic factor 1 (SF-1) is expressed in the adrenal cortex and gonads and regulates the expression of several P450 steroid hydroxylases in vitro. We examined the role of SF-1 in the adrenal glands and gonads in vivo by a targeted disruption of the mouse SF-1 gene. All SF-1-deficient mice died shortly after delivery. Their adrenal glands and gonads were absent, and persistent Mullerian structures were found in all genotypic males. While serum levels of corticosterone in SF-1-deficient mice were diminished, levels of adrenocorticotropic hormone (ACTH) were elevated, consistent with intact pituitary corticotrophs. Intrauterine survival of SF-1-deficient mice appeared normal, and they had normal serum level of corticosterone and ACTH, probably reflecting transplacental passage of maternal steroids. We tested whether SF-1 is required for P450 side-chain-cleavage enzyme (P450scc) expression in the placenta, which expresses both SF-1 and P450scc, and found that in contrast to its strong activation of the P450scc gene promoter in vitro, the absence of SF-1 had no effect on P450scc mRNA levels in vivo. Although the region targeted by our disruption is shared by SF-1 and by embryonal long terminal repeat-binding protein (ELP), a hypothesized alternatively spliced product, we believe that the observed phenotype reflects absent SF-1 alone, as PCR analysis failed to detect ELP transcripts in any mouse tissue, and sequences corresponding to ELP are not conserved across species. These results confirm that SF-1 is an important regulator of adrenal and gonadal development, but its regulation of steroid hydroxylase expression in vivo remains to be established.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J. Molecular control of cell fate in the neural crest: the sympathoadrenal lineage. Annu Rev Neurosci. 1993;16:129–158. doi: 10.1146/annurev.ne.16.030193.001021. [DOI] [PubMed] [Google Scholar]
- Bakke M., Lund J. Mutually exclusive interactions of two nuclear orphan receptors determine activity of a cyclic adenosine 3',5'-monophosphate-responsive sequence in the bovine CYP17 gene. Mol Endocrinol. 1995 Mar;9(3):327–339. doi: 10.1210/mend.9.3.7776979. [DOI] [PubMed] [Google Scholar]
- Ballard P. L. Hormonal regulation of pulmonary surfactant. Endocr Rev. 1989 May;10(2):165–181. doi: 10.1210/edrv-10-2-165. [DOI] [PubMed] [Google Scholar]
- Barnhart K. M., Mellon P. L. The orphan nuclear receptor, steroidogenic factor-1, regulates the glycoprotein hormone alpha-subunit gene in pituitary gonadotropes. Mol Endocrinol. 1994 Jul;8(7):878–885. doi: 10.1210/mend.8.7.7527122. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clemens J. W., Lala D. S., Parker K. L., Richards J. S. Steroidogenic factor-1 binding and transcriptional activity of the cholesterol side-chain cleavage promoter in rat granulosa cells. Endocrinology. 1994 Mar;134(3):1499–1508. doi: 10.1210/endo.134.3.8119192. [DOI] [PubMed] [Google Scholar]
- DEANE H. W., RUBIN B. L., DRIKS E. C., LOBEL B. L., LEIPSNER G. Trophoblastic giant cells in placentas of rats and mice and their probable role in steroid-hormone production. Endocrinology. 1962 Mar;70:407–419. doi: 10.1210/endo-70-3-407. [DOI] [PubMed] [Google Scholar]
- Durkee T. J., McLean M. P., Hales D. B., Payne A. H., Waterman M. R., Khan I., Gibori G. P450(17 alpha) and P450SCC gene expression and regulation in the rat placenta. Endocrinology. 1992 Mar;130(3):1309–1317. doi: 10.1210/endo.130.3.1537294. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Lala D. S., Luo X., Kim E., Moisan M. P., Parker K. L. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol. 1993 Jul;7(7):852–860. doi: 10.1210/mend.7.7.8413309. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Shen W. H., Ingraham H. A., Parker K. L. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol. 1994 May;8(5):654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Lala D. S., Ikeda Y., Luo X., Shen W. H., Nachtigal M. W., Abbud R., Nilson J. H., Parker K. L. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994 Oct 1;8(19):2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- Lala D. S., Rice D. A., Parker K. L. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992 Aug;6(8):1249–1258. doi: 10.1210/mend.6.8.1406703. [DOI] [PubMed] [Google Scholar]
- Lavorgna G., Ueda H., Clos J., Wu C. FTZ-F1, a steroid hormone receptor-like protein implicated in the activation of fushi tarazu. Science. 1991 May 10;252(5007):848–851. doi: 10.1126/science.1709303. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Tourtellotte L. C., Wesselschmidt R. L., Milbrandt J. Growth and differentiation proceeds normally in cells deficient in the immediate early gene NGFI-A. J Biol Chem. 1995 Apr 28;270(17):9971–9977. doi: 10.1074/jbc.270.17.9971. [DOI] [PubMed] [Google Scholar]
- Liggins G. C. Adrenocortical-related maturational events in the fetus. Am J Obstet Gynecol. 1976 Dec 1;126(7):931–941. doi: 10.1016/0002-9378(76)90680-3. [DOI] [PubMed] [Google Scholar]
- Luo X., Ikeda Y., Parker K. L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994 May 20;77(4):481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Lynch J. P., Lala D. S., Peluso J. J., Luo W., Parker K. L., White B. A. Steroidogenic factor 1, an orphan nuclear receptor, regulates the expression of the rat aromatase gene in gonadal tissues. Mol Endocrinol. 1993 Jun;7(6):776–786. doi: 10.1210/mend.7.6.8395654. [DOI] [PubMed] [Google Scholar]
- Michelsohn A. M., Anderson D. J. Changes in competence determine the timing of two sequential glucocorticoid effects on sympathoadrenal progenitors. Neuron. 1992 Mar;8(3):589–604. doi: 10.1016/0896-6273(92)90285-l. [DOI] [PubMed] [Google Scholar]
- Muglia L., Jacobson L., Dikkes P., Majzoub J. A. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995 Feb 2;373(6513):427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- Nomura M., Bärtsch S., Nawata H., Omura T., Morohashi K. An E box element is required for the expression of the ad4bp gene, a mammalian homologue of ftz-f1 gene, which is essential for adrenal and gonadal development. J Biol Chem. 1995 Mar 31;270(13):7453–7461. doi: 10.1074/jbc.270.13.7453. [DOI] [PubMed] [Google Scholar]
- Pakravan P., Kenny F. M., Depp R., Allen A. C. Familial congenital absence of adrenal glands; evaluation of glucocorticoid, mineralocorticoid, and estrogen metabolism in the perinatal period. J Pediatr. 1974 Jan;84(1):74–78. doi: 10.1016/s0022-3476(74)80556-1. [DOI] [PubMed] [Google Scholar]
- Pang S., Yang X., Wang M., Tissot R., Nino M., Manaligod J., Bullock L. P., Mason J. I. Inherited congenital adrenal hyperplasia in the rabbit: absent cholesterol side-chain cleavage cytochrome P450 gene expression. Endocrinology. 1992 Jul;131(1):181–186. doi: 10.1210/endo.131.1.1611996. [DOI] [PubMed] [Google Scholar]
- Shen W. H., Moore C. C., Ikeda Y., Parker K. L., Ingraham H. A. Nuclear receptor steroidogenic factor 1 regulates the müllerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994 Jun 3;77(5):651–661. doi: 10.1016/0092-8674(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Tsukiyama T., Niwa O., Yokoro K. Mechanism of suppression of the long terminal repeat of Moloney leukemia virus in mouse embryonal carcinoma cells. Mol Cell Biol. 1989 Nov;9(11):4670–4676. doi: 10.1128/mcb.9.11.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T., Ueda H., Hirose S., Niwa O. Embryonal long terminal repeat-binding protein is a murine homolog of FTZ-F1, a member of the steroid receptor superfamily. Mol Cell Biol. 1992 Mar;12(3):1286–1291. doi: 10.1128/mcb.12.3.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Sonoda S., Brown J. L., Scott M. P., Wu C. A sequence-specific DNA-binding protein that activates fushi tarazu segmentation gene expression. Genes Dev. 1990 Apr;4(4):624–635. doi: 10.1101/gad.4.4.624. [DOI] [PubMed] [Google Scholar]
- Ueda H., Sun G. C., Murata T., Hirose S. A novel DNA-binding motif abuts the zinc finger domain of insect nuclear hormone receptor FTZ-F1 and mouse embryonal long terminal repeat-binding protein. Mol Cell Biol. 1992 Dec;12(12):5667–5672. doi: 10.1128/mcb.12.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsicker K., Krisch B., Otten U., Thoenen H. Nerve growth factor-induced fiber outgrowth from isolated rat adrenal chromaffin cells: impairment by glucocorticoids. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3498–3502. doi: 10.1073/pnas.75.7.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. E., Fahrner T. J., Milbrandt J. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor-DNA interaction. Mol Cell Biol. 1993 Sep;13(9):5794–5804. doi: 10.1128/mcb.13.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F. A., Head J. R. Isolation and characterization of trophoblast from murine placenta. Placenta. 1986 Jul-Aug;7(4):349–364. doi: 10.1016/s0143-4004(86)80153-9. [DOI] [PubMed] [Google Scholar]