Figure 1. Central regulation of appetite and energy homoeostasis.
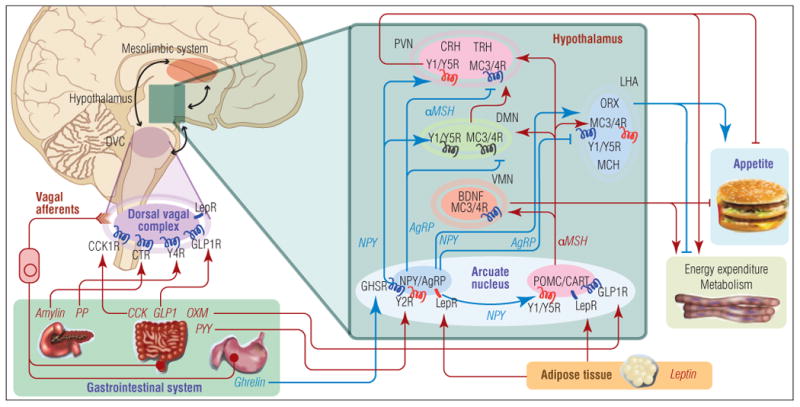
The hypothalamus is the major site of integration of anorexigenic and orexigenic signaling. Peripheral satiety hormones, such as ghrelin from the stomach and leptin from adipose tissue, primarily bind and activate their cognate receptors directly in the hypothalamus, particularly in the arcuate nucleus, or in the dorsal vagal complex in the medulla, which communicates with the hypothalamus. Among the neurons in the arcuate nucleus there exist two populations of neurons: those expressing the orexigenic neuropeptide Y (NPY) or agouti-related peptide (AgRP); and those expressing the anorexigenic pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART). Several satiety hormones induce their anorectic effects by either inhibiting the activity of NPY/AgRP neurons or activating POMC/CART neurons. These neurons in the arcuate nucleus project to second-order neurons in other hypothalamic nuclei, including the paraventricular nucleus (PVN), dorsomedial nucleus (DMN), ventromedial nucleus (VMN), and lateral hypothalamic area (LHA). These second-order hypothalamic neurons express anorexigenic neuropeptides (corticotropin-releasing hormone (CRH), thyrotropin-releasing hormone (TRH), brain-derived neurotrophic factor (BDNF)) and orexigenic neuropeptides (orexin (ORX), melanin-concentrating hormone (MCH)), which modulate appetite and energy homeostasis. Furthermore, the regulation of energy balance involves an integration of signaling from the hypothalamus, brainstem, and reward pathways of the mesolimbic system. Symbols: blue receptor = activating; red receptor = inhibiting; blue arrow = appetite-stimulating; red arrow = appetite-suppressing.
