Figure 3.
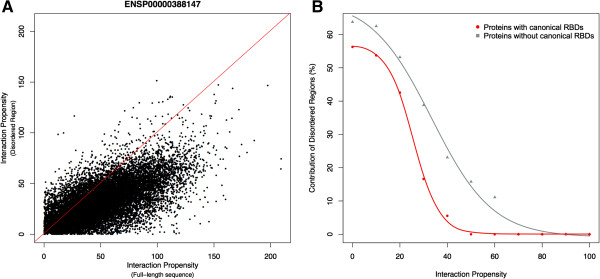
RNA-binding ability and structural disorder. (A) For each protein, we calculated RNA interactions with full-length sequences as well as structurally disordered regions [1,36]. When the interaction propensity score of a disordered region exceeds that of the full-length protein (points above the red line), disorder is considered to promote interaction with RNA molecules. (B) For 66% of the proteins (137 entries), disorder contributes at low interaction propensities, while full-length protein sequences dominate at high interaction propensities (Mann–Whitney U test). Overall, from low to high interaction propensities, the contribution of disorder decreases progressively with respect to that of the full-length protein (red and grey lines), in agreement with a previous analysis [25]. The role of disorder is more relevant in proteins lacking canonical RNA-binding domains (grey line), indicating that unstructured regions might have direct involvement in contacting RNA. Interaction propensities are averaged per protein. RBD, RNA-binding domain.
