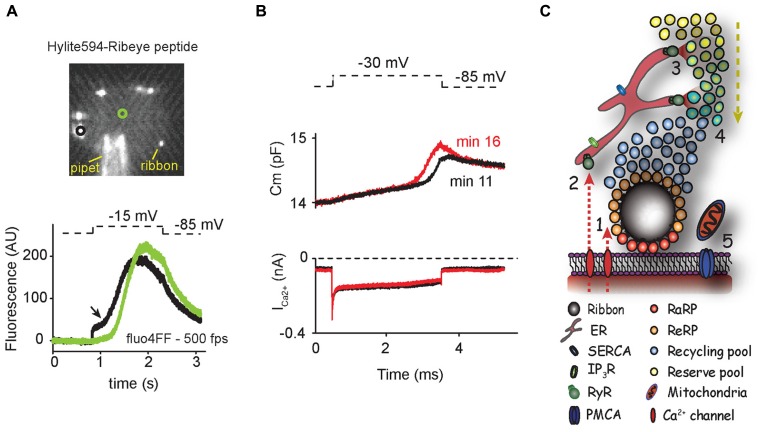
FIGURE 1.
(A) Hair cells show non-linear calcium increases during depolarization. Upper panel shows a swept-field confocal image of a single turtle auditory hair cell during patch clamp experiments. Hylite594-conjugated ribbon-binding peptide in the internal solution defined the location of synaptic ribbons for subsequent calcium imaging recordings (note that peptide also binds unspecifically to the pipet). In the lower panel, calcium levels were monitored in response to depolarization by swept-field confocal microscopy at 500 frames per second (fps) using the low-affinity calcium dye fluo4FF. Black and green traces correspond to intracellular locations near and far from synaptic ribbons (circles in upper panel). Arrow points the onset of supralinear calcium rise. (B) Hair cells show time-variant exocytic enhancement. In an independent experiment, calcium currents and real-time membrane capacitance were obtained by dual sine capacitance methods. Eleven minutes after whole cell configuration, a superlinear release was first obtained during prolonged moderate depolarization (black trace). The onset of superlinear release was shifted by equivalent calcium load 5 min later (red trace). (C) Intracellular calcium stores could modulate the recruitment of vesicles to the plasma membrane. Calcium influx through L-type calcium channels triggers exocytosis of synaptic vesicles near the active zone (1). In parallel, calcium also activates RyRs (2), triggering the release of calcium stored in the ER at sites far from the ribbon (3). CICR allows the recruitment of vesicles from reserve pools to the vicinity of the ribbon (4). (ER = endoplasmic reticulum; IP3R = inositol triphosphate receptor; SERCA = sarco/endoplasmic reticulum calcium ATPase; RaRP = rapid releasable pool; ReRP = readily releasable pool.) Mitochondria and calcium pumps maintain homeostatic calcium levels in cytoplasm (5). Arrows depict the direction of calcium influx and vesicle trafficking.