Figure 2.
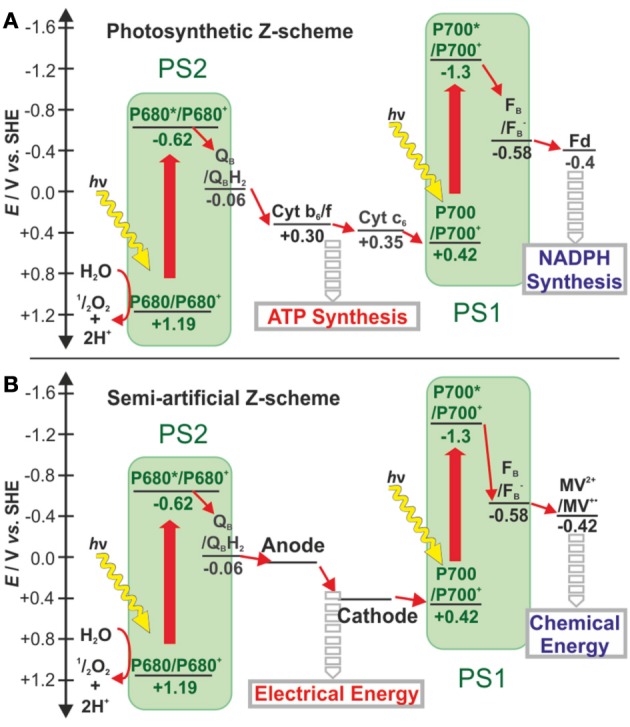
Schematic comparison of electron transfer chains in (A) natural photosynthesis and (B) semi-artificial photosynthesys diplaying the Z-scheme for the conversion of solar light to electrical and chemical energy - adapted from (Kothe et al., 2013a,b). Electron transfer steps are shown as small red arrows while light-induced charge separation steps are depicted as large red arrows. In natural photosynthesis, the electron transfer from QB in PS2 to P700 in PS1 creates a chemiosmotic potential further exploited for ATP synthesis. The high-energy electrons exiting PS1 are transferred via Fd to FNR for NAPD+ reduction to NADPH. In the semi-artificial Z-scheme the electron transfer chain between PS2 and PS1 is shortcut by electrodes to recover the energy as electricity. In addition, PS1 transfers its electrons to methyl viologen, an artificial charge carrier. The latter is envisioned to mediate these electrons to catalysts such as hydrogenases for chemical energy production (Haehnel and Hochheimer, 1979). Cyt c6: cytochrome c6, Cb6f:cytochrome b6f complex, Fd, ferredoxin; FNR, ferredoxin:NADP+ oxido reductase; MV2+, methyl viologen). Potentials are given in volt (V) versus the standard hydrogen electrode (SHE).
