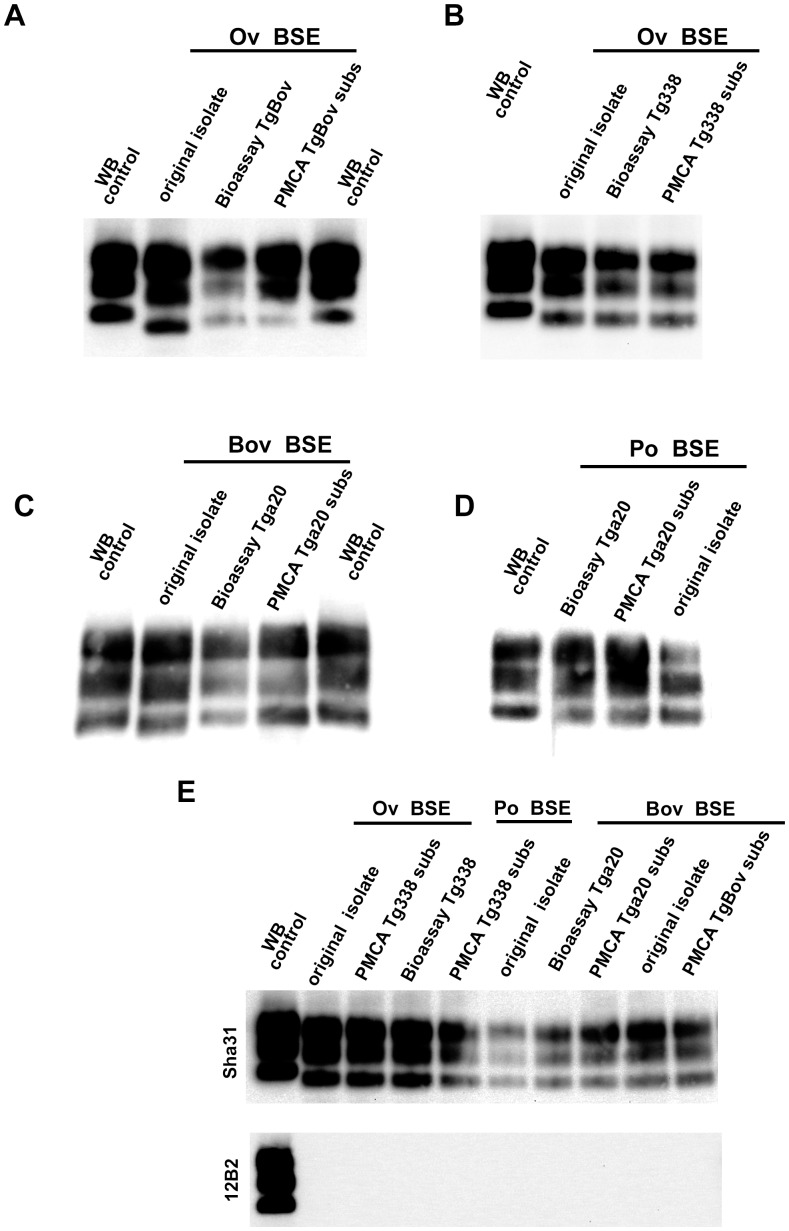
Figure 3. PrPres western blot (WB) profile of BSE originating from various species before and after PMCA amplification.
PMCA reactions were seeded with BSE brain material (10−2 dilution) from different species (bovine, sheep and porcine). PMCA substrates (sub) were prepared using brain from transgenic mice over-expressing the bovine (tgBov/tg110), the murine (tga20) or the sheep VRQ variant (tg338) Prion protein. PK digested abnormal PrP (PrPres) WB profile of (i) the original isolate used for seeding the PMCA reaction, (ii) the product of the PMCA reaction and (iii) the brain of a mouse intracerebrally inoculated with the seeding isolate and belonging to the line used to prepare the PMCA substrate, were compared. (A–D) WB PrPres signature using Sha31 anti PrP monoclonal antibody (epitope: YEDRYYRE, amino acid 145–152). (E) Relative immunoreactivity of Sha31 (epitope: YEDRYYRE, amino acid 145–152) and 12B2 (epitope: WGQGG, amino acid sequences 89–93) anti PrP monoclonal antibodies. In vCJD/BSE, the 12B2's epitope is cleaved by abnormal PrP PK digestion process. For this experiment, duplicate of each samples were submitted to abnormal PrP extraction before migration on two different gels and Western blotted. One of the WB membranes was probed with Sha31 while 12B2 was used for the second. On each gel a scrapie in sheep isolate was used as control (WB control).