…defining what level of nutrient intake is appropriate for a given GFR in men and women is very challenging because we do not know how to scale their GFR to their respective body compositions…
Keywords: body surface area, CKD complications, gender, GFR, uraemia
Abstract
Background
Men commence dialysis with a higher estimated glomerular filtration rate (eGFR) than women and are more likely to transition from chronic kidney disease (CKD) to end-stage renal disease. We hypothesized that for a given estimated body surface area (BSA) men have a greater metabolic burden, and that consequently, the practice of indexing GFR to BSA results in gender differences in the degree of biochemical uraemia.
Methods
Metabolic burden was assessed as estimated dietary protein, calorie, phosphorus, sodium and potassium intakes and urinary urea nitrogen excretion in the Chronic Renal Insufficiency Cohort, Modification of Diet in Renal Disease study, and National Health and Nutrition Examinations Surveys (NHANES) 1999–2010. Uraemia was characterized by serum biochemistry.
Results
Per m2 BSA, men had greater urea nitrogen excretion and intakes of all dietary parameters (P < 0.001 for all). For a given BSA-indexed iothalamate GFR or eGFR, male gender was associated with a 10–15% greater serum urea nitrogen (P < 0.001), giving men with a BSA-indexed GFR of 70–75 mL/min/1.73 m2 the same serum urea nitrogen concentration as women with a GFR of 60 mL/min/1.73 m2. However, indexing metabolic burden and GFR to alternative body size measures (estimated total body water, lean body mass or resting energy expenditure) abolished/reversed the gender associations. In NHANES, BSA-indexed eGFR distribution was very similar for men and women, so that adjusting for eGFR had little effect on the gender difference in serum urea.
Conclusions
Indexing GFR to BSA across genders may approximate nature's indexing approach, but gives men a greater ingested burden of protein, calories, sodium, phosphorus and potassium per mL/min GFR. This has implications for gender differences in CKD outcomes.
INTRODUCTION
Male gender is a predictor of adverse outcomes in chronic kidney disease (CKD); as in the general population, men with CKD have greater total and cardiovascular mortality than women at all levels of estimated glomerular filtration rate (eGFR) [1]. Men are also more likely to transition from CKD to end-stage renal disease [2] and commence dialysis at a higher average residual eGFR, suggesting an earlier onset of uraemic symptoms or biochemical complications [3, 4].
The degree of biochemical and clinical uraemia experienced by an individual with CKD partly reflects a balance between the level of residual excretory function and the rate of ingestion or endogenous production of a uraemic excretory burden (including phosphorus, sodium, potassium, acid and a number of poorly understood organic ‘toxins’ [5, 6]). On a per-weight basis, men ingest greater quantities of calories, protein, phosphorus, sodium and potassium than women [7, 8]. This is not surprising since women typically have a smaller percentage lean body mass (LBM) than men [9] and so a lower basal metabolic rate per unit weight [10]. Consequently, indexing GFR to weight might be expected to result in gender discrepancies in the degree of uraemia. Traditionally, eGFR is expressed indexed to an estimated body surface area (BSA) [11, 12], calculated from height and weight [13], although the rationale for this choice has been questioned [14]. The extent to which uraemic burden per m2 BSA differs between men and women with non-dialytic CKD has not previously been reported. Other measures of body size proposed for GFR indexing, such as estimated total body water (TBW) [15], resting energy expenditure (REE, i.e. basal metabolic rate) [10] or LBM [16–19], might better predict excretory burden. Notably, unlike estimated BSA, formula-based estimates of these parameters incorporate gender in recognition of the accompanying differences in body composition [10, 15, 16].
We hypothesized that for a given estimated BSA men have a greater uraemic excretory burden than women, resulting in a more advanced degree of biochemical uraemia for a given BSA-indexed eGFR. Using data from the Chronic Renal Insufficiency Cohort (CRIC), Modification of Diet in Renal Disease Study (MDRD) and US National Health and Nutrition Examination Surveys (NHANES) 1999–2010, gender differences in BSA-indexed metabolic burden were assessed in terms of estimated dietary intakes of protein, calories, phosphorus, sodium and potassium, and measured urinary urea nitrogen excretion. The effects of indexing GFR to alternative measures of body size on gender differences in serum urea and the likelihood of uraemic metabolic complications were also determined.
METHODS
Study populations
The recruitment procedures and characteristics of the CRIC, MDRD and NHANES cohorts have been published previously [20–24]. The CRIC is a longitudinal cohort study investigating the determinants of CKD progression and cardiovascular disease in CKD. The MDRD study was a randomized controlled trial of the effects of dietary protein restriction and blood pressure control on CKD progression. Baseline data from these two studies were used for the analysis of estimated dietary intakes, serum biochemistry, iothalamate-measured GFR (iGFR) and urine biochemistry. A small number (1%) of participants with 24-h urine creatinine of <350 or >3500 mg considered implausible [25] were excluded, leaving total cohorts of N = 3631 (CRIC) and N = 1679 (MDRD) with serum/urine biochemistry and body size data for analysis. Twenty-four hour urinary urea nitrogen results were available from both CRIC and MDRD, whereas urinary phosphorus, sodium and potassium measurements were only available from the MDRD dataset. Dietary intake estimates were complete for subsets of n = 2778 (CRIC) and n = 1267 (MDRD). Data from these studies were provided by the National Institutes for Diabetes and Digestive and Kidney Disease Data Repository [26] following Institutional Review Board Approval (University of Sheffield, UK).
The NHANES uses a multistage probability sampling design to create a sample representative of the non-institutionalized US population. Oversampling of certain population subgroups (non-Hispanic blacks, Mexican-Americans and the over 60s) increases the reliability of prevalence estimates in these groups [22]. For this analysis, data from the continuous NHANES cycles 1999–2010 were combined. Non-pregnant participants aged 20 years or older with complete dietary intake estimates and weight, height and serum biochemistry data were included in the analysis (N = 25 970). Associations between gender and metabolic burden were examined in NHANES subpopulations with an eGFR of <60 mL/min/1.73 m2 (n = 2472) and ≥60 mL/min/1.73 m2 (n = 23 498).
Dietary intake estimates
Dietary intakes of protein, calories, phosphorus, sodium and potassium were estimated using diet questionnaires and the University of Pittsburgh Nutrient Database [27] (MDRD) or the National Cancer Institute's Diet History Questionnaire [28] (CRIC). For NHANES participants, nutrient intakes were estimated with the USDA automated multi-pass 24-h dietary recall method and the Food and Nutrient Database for Dietary Studies [29].
Biochemistry analysis
Serum and urine biochemistry analyses were performed using standard bioanalyzers. Glomerular filtration rate was estimated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [12], with appropriate correction of the Beckman CX3-measured creatinines from MDRD [30]. Recalibration of serum creatinines across NHANES cycles was performed according to NHANES recommendations [31, 32]. Iothalamate clearance was used to measure GFR in MDRD and in a weighted random sample of one-third of CRIC participants. Analyses of the effects of GFR indexing measure on gender-associated differences in uraemic biochemistry included all subjects with demographic, biochemistry and iGFR measurements from CRIC (n = 1319) and MDRD (n = 1679). Hyperphosphataemia, hyperkalaemia and acidosis were defined as serum phosphorus of ≥4.6 mg/dL, potassium >5.0 mM and bicarbonate <22 mEq/L. Anaemia was defined both by the World Health Organization (WHO) gender-specific haemoglobin thresholds [33] (<13 and <12 g/dL for men and women, respectively) and the KDOQI definition [34] of <11 g/dL.
Body size indices
Body size estimates used for indexing metabolic burden and GFR as alternatives to BSA were weight, TBW, REE and LBM. Estimated BSA, TBW, REE and LBM were calculated using the formulae of Dubois and DuBois [13], Watson et al. [15], Mifflin et al. [10] and Deurenberg et al. [16], respectively. Fat-free mass was estimated in CRIC by bioimpedance analysis and was also tested as an indexing measure in this cohort.
Statistical analysis
Variables were naturally log transformed prior to parametric tests where appropriate. Gender differences in dietary intakes and urinary solute excretion rates indexed to alternative body size indices were examined by linear regression. Ratios between the geometric means for each gender were obtained by exponentiating the gender coefficients from linear regression models predicting log-transformed indexed dietary/urinary parameters.
The effects of indexing measured iGFR to alternative body size measures on gender differences in log-transformed serum urea nitrogen were also determined by linear regression. Log-transformation was applied to measures of GFR in these analyses to optimize both the normal distribution and the linearity of the relationship with log serum urea. The same approach was adopted to examine the effects of indexing GFR to alternative body size measures on gender differences in fractional excretions of phosphorus, sodium and potassium.
Odds ratios of hyperphosphataemia, hyperkalaemia, anaemia and acidosis associated with male versus female gender were determined in binary logistic regression models incorporating gender, age, race and indexed iGFR or eGFR. Whether indexing GFR to alternative body size indices improves complication prediction was assessed by calculating area under the receiver operating characteristic curve (C-statistic) and net reclassification index; subjects were ranked according to absolute GFR and to each indexed GFR, then the net reclassification according to the presence of each complication calculated as previously described [35].
In analyses adjusting for ethnicity, self-reported categories of non-Hispanic white, non-Hispanic black, Hispanic and ‘other’ were applied. Diabetes was also defined by self-report. All analyses performed using the NHANES data applied the appropriate sample weights according to NHANES recommended procedures [36]. Statistical analyses were performed using the SAS version 9.3, and SPSS version 20. A P-value of <0.05 was considered statistically significant.
RESULTS
BSA-indexed metabolic burden is greater in men
Characteristics of the MDRD and CRIC study participants with available dietary data are shown by gender in Table 1, together with those of the US population represented by NHANES 1999–2010 participants with an eGFR of <60 mL/min/1.73 m2. Of the alternative indices of body size considered (weight, BSA, TBW, REE and LBM) estimated BSA differed least between genders in all cohorts (ratios are shown in Supplementary Table S1). Consequently, gender differences in estimated dietary intakes were exaggerated when BSA rather than weight was used as the indexing measure (Table 2). The same pattern of results was found in NHANES participants with an eGFR of >60 mL/min/1.73 m2, though gender-associated differences in BSA-indexed intakes were generally greater in this population (Supplementary Table S2). Confining analyses to obese (body mass index, BMI, of ≥30 kg/m2) or non-obese subsets did not change the findings (not shown).
Table 1.
Demographic characteristics and dietary intakes of the study cohorts
| CRIC (N = 2778) |
MDRD (N = 1267) |
NHANES 1999–2010 (N = 2472) |
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| n (%) | 52.7 | 47.3 | 60.5 | 39.5 | 40.3 | 59.7 |
| Age, years, median (IQR) | 60.7 (13.3) | 59.9 (14.8) | 53.4 (21.1) | 50.5 (19.7) | 74.0 (15.0) | 75.0 (14.0) |
| Iothalamate GFR, mL/min, median (IQR)a | 59.0 (33.6) | 51.6 (30.3) | 39.5 (27.1) | 32.3 (21.8) | N/A | N/A |
| eGFR, mL/min/1.73 m2, median (IQR) | 44.7 (20.5) | 43.2 (21.8) | 35.7 (22.8) | 35.2 (21.6) | 51.8 (14.1) | 50.3 (14.2) |
| BMI, kg/m2, mean (SD) | 30.9 (6.0) | 33.1 (9.2) | 27.5 (4.1) | 26.6 (5.4) | 28.7 (5.5) | 29.2 (6.7) |
| Weight, kg, mean (SD)b | 95.4 (20.8) | 87.3 (25.0) | 85.7 (14.8) | 70.1 (14.8) | 87.0 (19.1) | 74.2 (19.1) |
| Estimated BSA, m2, mean (SD)b | 2.14 (0.25) | 1.97 (0.29) | 2.02 (0.19) | 1.74 (0.17) | 2.01 (0.22) | 1.76 (0.22) |
| TBW, L, mean (SD)b | 47.9 (7.5) | 36.6 (6.4) | 45.5 (5.6) | 32.6 (3.9) | 43.9 (7.3) | 33.2 (5.1) |
| REE, kcal/24 h, mean (SD)b | 1760 (240) | 1440 (270) | 1710 (190) | 1310 (170) | 1610 (240) | 1220 (240) |
| LBM, kg, mean (SD)b | 61.1 (7.7) | 43.1 (6.2) | 60.5 (7.5) | 42.6 (5.1) | 56.0 (8.1) | 43.5 (6.3) |
| UUN, g, median (IQR)b | 8.80 (5.50) | 6.84 (3.87) | 9.60 (3.90) | 7.40 (3.10) | N/A | N/A |
| Protein, g/24 h, median (IQR)b | 69.9 (46.1) | 56.5 (36.6) | 90.9 (35.3) | 67.0 (25.8) | 74.8 (43.2) | 53.8 (32.3) |
| Phosphorus, mEq/24 h, median (IQR)b | 1150 (710) | 950 (630) | 1390 (530) | 1050 (410) | 1210 (700) | 890 (520) |
| Energy, kcal/24 h, median (IQR)b | 1820 (1090) | 1480 (940) | 2300 (770) | 1700 (580) | 1840 (990) | 1410 (740) |
| Sodium, mEq/24 h median (IQR)b | 2870 (1840) | 2310 (1480) | 3460 (1710) | 2640 (1230) | 2690 (1710) | 2250 (1400) |
| Potassium, mEq/24 h, median (IQR)b | 3010 (1740) | 2500 (1620) | 3310 (1220) | 2570 (1060) | 2690 (1500) | 2040 (1190) |
Characteristics are shown for the CRIC and MDRD subpopulations with complete dietary data and for the US population represented by NHANES 1999–2010 participants with an eGFR of <60 mL/min/1.73 m2.
aAbsolute iothalamate GFR was measured in a CRIC subpopulation of n = 1319.
bP < 0.001 between genders in all cohorts.
Table 2.
Influence of the choice of indexing measure on gender differences in estimated dietary intakes
| Indexed to | CRIC (N = 2778) |
MDRD (N = 1267) |
NHANES (N = 2472) |
||||
|---|---|---|---|---|---|---|---|
| Ratio (95% CI) | P-value | Ratio (95% CI) | P-value | Ratio (95% CI) | P-value | ||
| Protein | Weight | 1.14 (1.10–1.18) | <0.001 | 1.11 (1.03–1.15) | <0.001 | 1.18 (1.12–1.25) | <0.001 |
| BSA | 1.16 (1.12–1.20) | <0.001 | 1.18 (1.13–1.22) | <0.001 | 1.22 (1.16–1.28) | <0.001 | |
| TBW | 0.96 (0.93–1.00) | 0.03 | 0.98 (0.94–1.01) | 0.19 | 1.06 (1.01–1.11) | <0.02 | |
| REE | 1.02 (0.99–1.06) | 0.21 | 1.04 (1.00–1.08) | 0.07 | 1.05 (1.00–1.11) | <0.04 | |
| LBM | 0.89 (0.86–0.92) | <0.001 | 0.96 (0.92–0.99) | 0.02 | 0.97 (0.92–1.02) | 0.18 | |
| Calories | Weight | 1.12 (1.08–1.15) | <0.001 | 1.11 (1.08–1.15) | <0.001 | 1.13 (1.08–1.18) | <0.001 |
| BSA | 1.13 (1.10–1.17) | <0.001 | 1.18 (1.14–1.22) | <0.001 | 1.16 (1.11–1.21) | <0.001 | |
| TBW | 0.94 (0.91–0.97) | <0.001 | 0.98 (0.95–1.01) | 0.18 | 1.01 (0.96–1.05) | 0.64 | |
| REE | 1.00 (0.97–1.03) | 0.89 | 1.04 (1.01–1.07) | 0.02 | 1.00 (0.96–1.05) | 0.83 | |
| LBM | 0.87 (0.84–0.90) | <0.001 | 0.96 (0.93–0.99) | 0.01 | 0.92 (0.88–0.96) | <0.001 | |
| Phosphate | Weight | 1.09 (1.05–1.13) | <0.001 | 1.08 (1.04–1.12) | <0.001 | 1.15 (1.09–1.21) | <0.001 |
| BSA | 1.11 (1.07–1.14) | <0.001 | 1.15 (1.11–1.19) | <0.001 | 1.18 (1.13–1.24) | <0.001 | |
| TBW | 0.92 (0.89–0.95) | <0.001 | 0.95 (0.92–0.99) | 0.01 | 1.03 (0.98–1.08) | 0.23 | |
| REE | 0.98 (0.94–1.01) | 0.16 | 1.01 (0.97–1.05) | 0.53 | 1.02 (0.98–1.07) | 0.33 | |
| LBM | 0.85 (0.82–0.88) | <0.001 | 0.93 (0.90–0.97) | <0.001 | 1.06 (1.01–1.12) | 0.01 | |
| Sodium | Weight | 1.13 (1.09–1.17) | <0.001 | 1.01 (0.96–1.05) | 0.72 | 1.14 (1.08–1.21) | <0.001 |
| BSA | 1.15 (1.12–1.19) | <0.001 | 1.07 (1.02–1.12) | 0.003 | 1.17 (1.12–1.24) | <0.001 | |
| TBW | 0.96 (0.93–0.99) | 0.02 | 0.89 (0.85–0.93) | <0.001 | 1.02 (0.97–1.08) | 0.56 | |
| REE | 1.02 (0.99–1.05) | 0.27 | 0.94 (0.90–0.99) | 0.009 | 1.02 (0.97–1.07) | <0.001 | |
| LBM | 0.89 (0.86–0.92) | <0.001 | 0.87 (0.83–0.91) | <0.001 | 0.93 (0.89–0.98) | 0.10 | |
| Potassium | Weight | 1.05 (1.01–1.09) | 0.009 | 0.99 (0.96–1.03) | 0.68 | 1.10 (1.04–1.15) | <0.001 |
| BSA | 1.07 (1.03–1.10) | <0.001 | 1.05 (1.02–1.09) | 0.004 | 1.13 (1.08–1.18) | <0.001 | |
| TBW | 0.89 (0.86–0.92) | <0.001 | 0.87 (0.84–0.90) | <0.001 | 1.02 (0.94–1.03) | <0.001 | |
| REE | 0.94 (0.91–0.97) | <0.001 | 0.93 (0.90–0.96) | <0.001 | 1.02 (0.93–1.02) | <0.001 | |
| LBM | 0.82 (0.79–0.85) | <0.001 | 0.86 (0.83–0.89) | <0.001 | 0.90 (0.86–0.94) | <0.001 | |
Male:female ratios for geometric means of indexed estimated dietary intakes are shown, adjusted for age and race.
BSA, estimated body surface area; TBW, estimated total body water; REE, estimated resting energy expenditure; LBM, estimated lean body mass.
Indexing to TBW, REE or LBM rather than BSA attenuated or reversed the gender-associated differences in dietary intakes; overall, the greatest disparities in dietary intakes associated with gender were apparent when indexed to BSA (though all indexing measures reduced the gender differences compared with those seen between absolute dietary intakes). When bioimpedance-measured fat-free mass was applied as the indexing measure in CRIC, all dietary intakes were significantly greater in women (Supplementary Table S3).
When indexed to weight or BSA, 24-h urinary urea nitrogen excretion (thus generation) rate was significantly greater in men (Table 3). Indexing to TBW or LBM reversed the gender difference, whereas urea nitrogen excretion indexed to REE did not differ between men and women. Results of 24-h urinary phosphorus, sodium and potassium measurements were available from MDRD participants and also showed a significantly greater excretory burden in men when indexed to BSA (P < 0.001 for all), but not when indexed to TBW, REE or LBM (Supplementary Table S4). Inclusion of data from the small percentage of subjects considered to have implausible values for creatininuria had no effect on these conclusions (not shown).
Table 3.
Influence of indexing measure on gender differences in urine urea nitrogen excretion rate
| Indexed to: | CRIC (N = 3631) |
MDRD (N = 1679) |
|||
|---|---|---|---|---|---|
| Ratio (95%CI) | P-value | Ratio | P-value | ||
| 24 h urine urea nitrogen | Weight | 1.11 (1.08–1.15) | <0.001 | 1.04 (1.01–1.07) | 0.018 |
| BSA | 1.13 (1.10–1.17) | <0.001 | 1.10 (1.07–1.13) | <0.001 | |
| TBW | 0.94 (0.91–0.97) | <0.001 | 0.91 (0.88–0.94) | <0.001 | |
| REE | 1.00 (0.97–1.03) | 1.00 | 0.97 (0.94–1.00) | 0.03 | |
| LBM | 0.87 (0.84–0.90) | <0.001 | 0.89 (0.87–0.92) | <0.001 | |
Male: female ratios for geometric means of indexed measured urinary urea nitrogen excretion rates are shown, adjusted for age and race.
BSA, estimated body surface area; TBW, estimated total body water; REE, estimated resting energy expenditure; LBM, estimated lean body mass.
Indexing GFR to BSA results in gender differences in serum urea nitrogen
There was a negative linear relationship between log-transformed serum urea nitrogen and log-transformed GFR, consistent with the expected reciprocal relationship between GFR and serum urea nitrogen. Ratios between the geometric mean serum urea nitrogen concentrations for men and women, adjusted for age, race and log-transformed absolute or indexed GFR are summarized in Table 4. When GFR was indexed to BSA, male gender remained independently associated with greater log serum urea nitrogen. Adjustment for diabetes status and 24-h urine volume (as a measure of hydration status) or confining the analyses to non-obese participants (BMI of <30 kg/m2) did not materially alter these findings. Further adjustment for diuretic use and a diagnosis of congestive cardiac failure (data only available in CRIC) also did not change the conclusions. Based on these regression results, women with a BSA-indexed iGFR of 60 mL/min/1.73 m2 had the same mean log serum urea nitrogen as men with BSA-indexed GFRs of 75 and 70 mL/min/1.73 m2 in CRIC and MDRD, respectively. Equivalent male GFRs corresponding to a female BSA-indexed iGFR of 20 mL/min/1.73 m2 were 25 and 23 mL/min/1.73 m2. Similar findings were apparent with application of a BSA-indexed eGFR. Indexing GFR to TBW, REE or LBM rather than BSA attenuated or reversed the association between log serum urea nitrogen and gender. The relationship between gender and serum urea nitrogen in CRIC is shown for the various indexing approaches in Figure 1.
Table 4.
Effects of GFR indexing measure on gender differences in serum urea nitrogen concentration
| GFR measure | CRIC |
MDRD |
||
|---|---|---|---|---|
| Ratio (95% CI) | P-value | Ratio (95% CI) | P-value | |
| Absolute iGFR | 1.22 (1.18–1.27) | <0.001 | 1.23 (1.20–1.26) | <0.001 |
| iGFR/weight | 1.14 (1.10–1.18) | <0.001 | 1.07 (1.04–1.10) | <0.001 |
| iGFR/BSA | 1.15 (1.12–1.19) | <0.001 | 1.12 (1.09–1.14) | <0.001 |
| iGFR/TBW | 1.02 (0.99–1.06) | 0.25 | 0.98 (0.95–1.00) | 0.10 |
| iGFR/REE | 1.06 (1.03–1.10) | <0.001 | 1.02 (1.00–1.05) | 0.10 |
| iGFR/LBM | 0.99 (0.95–1.02) | 0.45 | 0.97 (0.94–0.99) | 0.01 |
| eGFR | 1.12 (1.10–1.14) | <0.001 | 1.10 (1.07–1.13) | <0.001 |
Ratios between the geometric mean serum urea nitrogen concentrations in men and women are shown, adjusted for age, race and the indicated indexed iothalamate GFR or calculated eGFR.
iGFR, iothalamate GFR; BSA, estimated body surface area; TBW, estimated total body water; REE, estimated resting energy expenditure; LBM, estimated lean body mass.
FIGURE 1:
Effects of the choice of GFR indexing measure on the relationship between gender and serum urea nitrogen in the CRIC cohort. Serum urea nitrogen versus: (A) eGFR, (B) iGFR indexed to weight, (C) iGFR indexed to BSA, (D) iGFR indexed to TBW, (E) iGFR indexed to REE and (F) iGFR indexed to LBM. Loess-smoothed plots are shown. (For comparison of the effects of gender across the respective ranges of excretory function, curves versus indexed iothalamate GFR for women are truncated with exclusion of three outlying high GFR datapoints). iGFR, iothalamate GFR; BSA, estimated body surface area; TBW, estimated total body water; REE, estimated resting energy expenditure; LBM, estimated lean body mass.
In the NHANES cohort (where iGFR was not measured), male gender was associated with a greater log serum urea nitrogen, independently of log eGFR (P < 0.001). The male:female ratio of geometric mean serum urea concentrations, adjusted for log eGFR, age and race, was 1.14 (95% CI 1.13–1.15, P < 0.001). Similar significant differences between genders were evident in subpopulations defined by excretory impairment (geometric mean ratios of 1.13 and 1.14 for subjects with an eGFR of <60 and ≥60, respectively) or obesity (ratios of 1.16 and 1.14 for BMI of <30 and ≥30 kg/m2, respectively) and were not affected by adjustment for a diagnosis of cardiac failure. Male eGFRs corresponding to the same mean log serum urea nitrogen concentrations as eGFRs of 60 and 20 mL/min/1.73 m2 in women were 74 and 25 mL/min/1.73 m2, respectively.
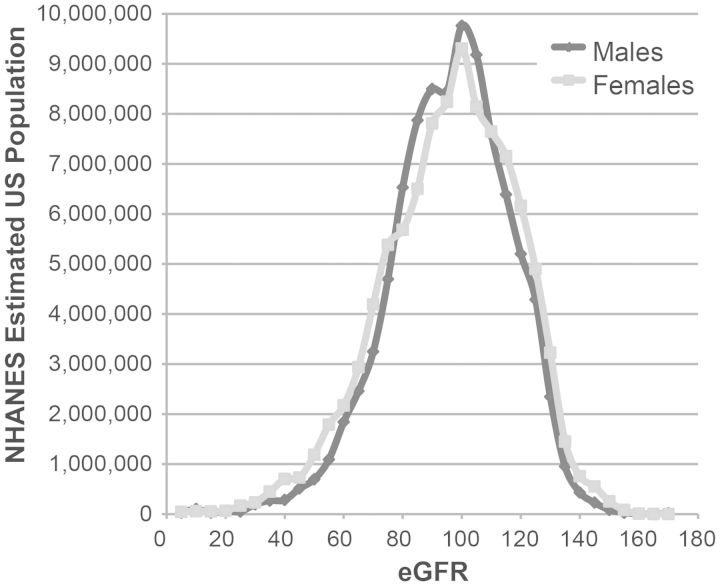
BSA-indexed eGFR reflects natural GFR scaling equally across genders
To determine whether BSA-indexed eGFR reflects the ‘natural’ scaling of GFR (i.e. the relationship between absolute GFR endowment and body size) equally in men/women, the US adult population distribution of eGFR by gender was examined in NHANES 1999–2010. The mean eGFR did not differ by gender (94 mL/min/1.73 m2 for both), but the distribution of eGFR was slightly more widely spread in females (Figure 2). Consistent with the similar distribution of eGFR in men and women, eGFR adjustment had little effect on the gender difference in log serum urea: the male:female ratio of geometric mean serum urea concentrations, adjusted just for age and race, was 1.16 (95% CI 1.15–1.17, P < 0.001) c.f. 1.14 with eGFR adjustment (as above). Therefore, the body size measure to which GFR naturally scales seems to match urea generation rate to excretory function across genders no better than BSA-indexing.
FIGURE 2:
The distribution of eGFR by gender in NHANES 1999–2010.
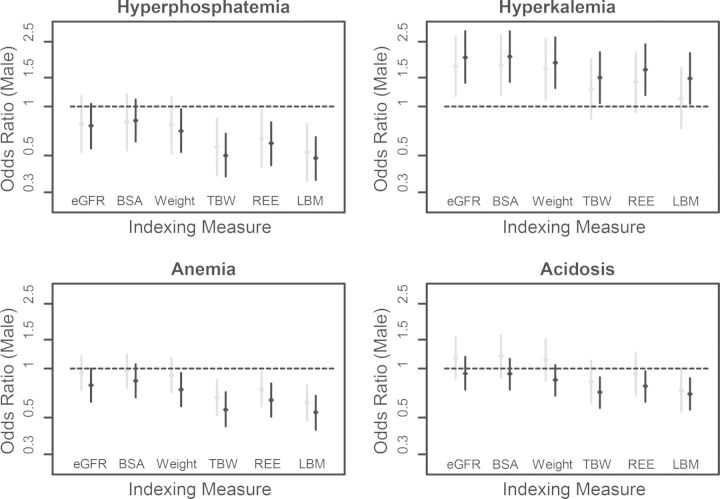
Choice of indexing measure and gender differences in CKD complication risk
Indexing iGFR to TBW, REE or LBM rather than BSA predictably reduced the ORs associated with male gender for all complications (Figure 3). Consequently, an association between male gender and greater likelihood of hyperkalaemia was attenuated, whereas significant associations were revealed between male gender and lower likelihoods of hyperphosphataemia and WHO-defined anaemia. Using a single haemoglobin cut-off of < 11 g/dL resulted in a lower likelihood of anaemia in men when BSA-indexed GFR was applied (OR 0.37 and 0.42 in MDRD and CRIC, respectively, P < 0.001 for both), but the OR was further reduced with the alternative indexing measures.
FIGURE 3:
ORs of CKD complications by male gender in models with eGFR or with iothalamate GFR indexed to alternative body size measures. ORs with 95% confidence interval are shown for men relative to women in the CRIC (grey lines) and MDRD (black lines) cohorts, adjusted for age and race. Complications defined as: hyperphosphataemia, serum phosphorus ≥ 4.6 mg/dL; hyperkalaemia, serum potassium > 5.0 mM; acidosis, serum bicarbonate < 22 mEq/L; anaemia, haemoglobin < 13 and <12 g/dL for men and women, respectively.
In linear regression models, adjusting for age and log-transformed indexed GFR, men had a greater fractional excretion of phosphorus than women, regardless of the GFR indexing measure applied (P < 0.001 for all, Supplementary Table S5). However, consistent with the greater dietary phosphorus burden per m2 BSA in men, the gender difference in fractional phosphorus excretion was greatest when BSA-indexed GFR was used (ratio of geometric means 1.23, 95% CI 1.19–1.27). A greater fractional excretion of sodium was also evident in men for a given BSA-indexed GFR (ratio of geometric means 1.12, 95% CI 1.08–1.17).
Compared with absolute (unindexed) iGFR, indexing GFR to any measure of body size failed to demonstrate an improvement in the prediction of hyperphosphataemia, acidosis, anaemia or hyperkalaemia by C-statistic or net reclassification index (not shown).
DISCUSSION
It is only logical to index GFR to some measure of metabolic burden or body size for two reasons: first, indexing excretory function to metabolic burden should improve the consistency of prediction of CKD complications and requirement for dialysis. Secondly, since absolute GFR is normally greater in bigger people [17, 19, 37–39], expressing GFR indexed to body size should facilitate the detection of an ‘abnormal’ reduction in GFR. Whether BSA is the optimal indexing measure for either of these two objectives has not been determined; we examined the implications of BSA-indexing on gender differences in uraemic biochemistry.
Our work demonstrates that men have a greater ingested burden of protein, calories, phosphorus, sodium and potassium per m2 BSA. In fact, compared with weight-indexing, BSA-indexing exaggerates gender differences in dietary intakes and in the urea generation rate. Consequently, for a given BSA-indexed GFR, men have a greater ingested dietary burden and urea generation rate per mL/min GFR. Indexing GFR to BSA, thus, results in a significantly greater serum urea nitrogen concentration in men, which is not apparent when indexing to plausible alternative measures of body size.
Since urea is probably not a major driver of most of the clinical phenomena that constitute the syndrome of uraemia [6], gender differences in serum urea nitrogen concentration have not attracted much interest. However, urea has postulated toxic properties that could contribute to adverse outcomes [40–42], and greater serum urea nitrogen concentrations may be accompanied by greater levels of unmeasured uraemic toxins derived from catabolism of nitrogenous gut contents [43]. The greater calorie consumption per m2 BSA in men also might be accompanied by increased concentrations of unmeasured uraemic by-products of energy metabolism at a given BSA-indexed GFR. Gender differences in intakes of sodium, phosphorus and potassium per mL/min GFR at a given BSA-indexed GFR are potentially clinically important; dietary restriction of these substances is commonly required in advanced CKD to prevent clinical/biochemical complications (though guideline dietary restriction targets are not gender-specific [44]).
The gender-associated urea nitrogen concentration difference at any given BSA-indexed GFR/eGFR is small in absolute terms, but when considered as a marker of the degree of accompanying general uraemic burden it is potentially of significant epidemiological consequence. Men with an eGFR of 70–75 mL/min/1.73 m2 have approximately the same serum urea nitrogen as women with an eGFR of 60 mL/min/1.73 m2; men may therefore experience a pathological uraemic biochemical milieu at a higher BSA-indexed GFR than women. At low levels of BSA-indexed GFR/eGFR, the difference is equivalent to a few mL/min/1.73 m2, which would be sufficient to explain the reported earlier start of dialysis in men [3, 4]. We can also speculate that gender differences in the balance between ingested burden (e.g. of sodium), and GFR might contribute to increased total/cardiovascular mortality in men even at the earliest stages of CKD.
In the US population represented by NHANES 1999–2010, average BSA-indexed eGFR was the same for both genders, so that the gender difference in serum urea nitrogen was largely unaffected by eGFR adjustment. Some studies in healthy potential kidney donors have reported that measured GFR scales to BSA similarly in both genders [19, 37], whereas others have found GFR/BSA to be slightly greater in men [38, 39]. If GFR does, indeed, naturally scale closely to BSA across genders, then this seems to be an unfortunate circumstance for men; the consequence is a greater natural average excretory burden of urea, sodium, phosphorus and potassium per mL/min GFR in men. Men would need a greater increment in absolute GFR endowment (such as in proportion to their greater LBM or TBW) in order to match their greater ingested burden.
Since, as we demonstrate, indexing GFR to TBW or LBM abolishes gender differences in serum urea, the observed greater serum urea nitrogen concentration in NHANES men suggests that GFR does not naturally scale to TBW or LBM across genders. Consequently, with regard to the derivation and clinical application of an alternative eGFR indexing measure, indexing to LBM or TBW would better match excretory burden to function by gender, perhaps predicting dialysis initiation more consistently in men/women; however, this approach would require gender-specific definitions of ‘normal’ eGFR ranges.
Gender differences in CKD complication risk are not simply a consequence of the differential scaling of ingested burden and GFR, but also reflect differences in physiology. For example, higher serum phosphorus levels have previously been reported in women with CKD [45] and may reflect sex hormone effects on renal phosphorus handling [46, 47]. Consequently, indexing GFR to body size measures that better predict ingested burden does not abolish gender differences in complication risk, but rather creates a significant gender difference in the likelihood of hyperphosphataemia. Gender-associated CKD physiology differences thus may not be apparent if differences in ingested burden are not accounted for.
Our work has some limitations: the MDRD and CRIC study cohorts are selected populations and dietary records were not available for all participants. A selection bias may, therefore, have influenced the conclusions. However, our findings are consistent across both these cohorts despite different participant characteristics, as well as in the analysis of NHANES 1999–2010, which uses sample weights to account for incomplete or non-response to dietary intake questionnaires. Gender might influence serum urea nitrogen independently of the balance between excretory burden and excretory function, through confounding-associated differences in tubular urea handling or extrarenal urea hydrolysis [48]. However, indexing GFR to body size measures that show the same association with the urea excretion rate or protein intake in both genders abolishes the gender difference in serum urea. This strongly suggests that differences in BSA-indexed protein ingestion and urea generation rather than unrecognized confounders are responsible for the greater serum urea nitrogen in men at a given BSA-indexed GFR.
In conclusion, we demonstrate that although the US population average BSA-indexed eGFR is the same in men and women, gender differences in ingested metabolic burden are most evident when indexed to BSA. Men thus seem to be naturally endowed with less excretory function for a given ingested burden of protein, calories, phosphorus, sodium and potassium. The greater ingested burden per mL/min GFR at a given BSA-indexed GFR has implications for observed gender differences in CKD outcomes; future characterization of novel uraemic toxins should reveal whether men experience a pathological uraemic milieu at an earlier stage of CKD.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
All authors have no conflicts of interest to declare. The results presented in this paper have not been published previously in whole or part. (See related article by Nitsch. Is there a difference in metabolic burden between men and women? Nephrol Dial Transplant 2014; 29: 1110–1112.)
Supplementary Material
ACKNOWLEDGEMENTS
The MDRD and CRIC studies were conducted by the MDRD and CRIC Study Investigators, respectively, and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data from the MDRD and CRIC studies reported here were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with Investigators of the MDRD or CRIC studies and does not necessarily reflect the opinions or views of the MDRD/CRIC Investigators, the NIDDK Central Repositories or the NIDDK. The authors thank the NHANES participants, staff and investigators. T.E. is supported by Clinical Research Fellowships from the British Heart Foundation and National Institute for Healthcare Research, UK. J.F. is supported by a Clinical Research Fellowship from Kidney Research UK.
REFERENCES
- 1.Nitsch D, Grams M, Sang Y, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis. BMJ. 2013;346:f324. doi: 10.1136/bmj.f324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grams ME, Chow EK, Segev DL, et al. Lifetime incidence of CKD Stages 3–5 in the United States. Am J Kidney Dis. 2013;62:245–352. doi: 10.1053/j.ajkd.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright S, Klausner D, Baird B, et al. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol. 2010;5:1828–1835. doi: 10.2215/CJN.06230909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molnar MZ, Streja E, Kovesdy CP, et al. Estimated glomerular filtration rate at reinitiation of dialysis and mortality in failed kidney transplant recipients. Nephrol Dial Transplant. 2012;27:2913–2921. doi: 10.1093/ndt/gfs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanholder R, Baurmeister U, Brunet P, et al. A bench to bedside view of uremic toxins. J Am Soc Nephrol. 2008;19:863–870. doi: 10.1681/ASN.2007121377. [DOI] [PubMed] [Google Scholar]
- 6.Meyer TW, Hostetter TH. Uremia. N Engl J Med. 2007;357:1316–1325. doi: 10.1056/NEJMra071313. [DOI] [PubMed] [Google Scholar]
- 7.Moore LW, Byham-Gray LD, Scott Parrott J, et al. The mean dietary protein intake at different stages of chronic kidney disease is higher than current guidelines. Kidney Int. 2013;83:724–732. doi: 10.1038/ki.2012.420. [DOI] [PubMed] [Google Scholar]
- 8.Kopple JD, Greene T, Chumlea WC, et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int. 2000;57:1688–1703. doi: 10.1046/j.1523-1755.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 9.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;(6):60–75. doi: 10.1016/j.genm.2009.02.002. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mifflin MD, St Jeor ST, Hill LA, et al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51:241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DuBois D, DuBois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 14.Delanaye P, Mariat C, Cavalier E, et al. Errors induced by indexing glomerular filtration rate for body surface area: reduction ad absurdum. Nephrol Dial Transplant. 2009;24:3593–3596. doi: 10.1093/ndt/gfp431. [DOI] [PubMed] [Google Scholar]
- 15.Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- 16.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105–114. doi: 10.1079/bjn19910073. [DOI] [PubMed] [Google Scholar]
- 17.Eriksen BO, Melsom T, Mathisen UD, et al. GFR normalized to total body water allows comparisons across genders and body sizes. J Am Soc Nephrol. 2011;22:1517–1525. doi: 10.1681/ASN.2010121321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boer P. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am J Physiol. 1984;247(4 Pt 2):F632–F636. doi: 10.1152/ajprenal.1984.247.4.F632. [DOI] [PubMed] [Google Scholar]
- 19.Daugirdas JT, Meyer K, Greene T, et al. Scaling of measured glomerular filtration rate in kidney donor candidates by anthropometric estimates of body surface area, body water, metabolic rate, or liver size. Clin J Am Soc Nephrol. 2009;4:1575–1583. doi: 10.2215/CJN.05581008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beck GJ, Berg RL, Coggins CH, et al. Design and statistical issues of the Modification of Diet in Renal Disease Trial. The Modification of Diet in Renal Disease Study Group. Control Clin Trials. 1991;12:566–586. doi: 10.1016/0197-2456(91)90069-x. [DOI] [PubMed] [Google Scholar]
- 21.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services; Centers for Disease Control and Prevention. National Health and Nutrition examination Survey http://www.cdc.gov/nchs/nhanes.htm. (February 21 2013, date last accessed) [Google Scholar]
- 23.Buckalew VM, Jr, Berg RL, Wang SR, et al. Prevalence of hypertension in 1,795 subjects with chronic renal disease: the modification of diet in renal disease study baseline cohort. Modification of Diet in Renal Disease Study Group. Am J Kidney Dis. 1996;28:811–821. doi: 10.1016/s0272-6386(96)90380-7. [DOI] [PubMed] [Google Scholar]
- 24.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ix JH, Wassel CL, Stevens LA, et al. Equations to estimate creatinine excretion rate: the CKD epidemiology collaboration. Clin J Am Soc Nephrol. 2011;6:184–191. doi: 10.2215/CJN.05030610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NIDDK Central Repository. http://www.niddkrepository.org/niddk/home.do. (21st January 2013, , date last accessed)
- 27.Gillis BP, Averbach FM, Caggiula AW, et al. Features of the nutrient database and analysis system for the Modification of Diet in Renal Disease Study. Control Clin Trials. 1994;15:44–58. doi: 10.1016/0197-2456(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 28.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 29. http://www.ars.usda.gov/ba/fsrg. (13 May 2013, date last accessed)
- 30.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 31.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/nhanes2005–2006/BIOPRO_D.htm#Analytic_Notes. (21 January 2013, date last accessed) [Google Scholar]
- 33.WHO. Geneva: World Health Organization; 1968. Nutritional Anemia, Report of a World Health Organisation Scientific Group. [Google Scholar]
- 34.KDOQI clinical practice guideline and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471–530. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. NHANES Analytic Guidelines. 2010 http://www.cdc.gov/nchs/nhanes/nhanes2003–2004/analytical_guidelines.htm. (21st January 2013, date last accessed)
- 37.Grewal GS, Blake GM. Reference data for 51Cr-EDTA measurements of the glomerular filtration rate derived from live kidney donors. Nucl Med Commun. 2005;26:61–65. doi: 10.1097/00006231-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Visser FW, Muntinga JH, Dierckx RA, et al. Feasibility and impact of the measurement of extracellular fluid volume simultaneous with GFR by 125I-iothalamate. Clin J Am Soc Nephrol. 2008;3:1308–1315. doi: 10.2215/CJN.05501207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters AM, Perry L, Hooker CA, et al. Extracellular fluid volume and glomerular filtration rate in 1878 healthy potential renal transplant donors: effects of age, gender, obesity and scaling. Nephrol Dial Transplant. 2012;27:1429–1437. doi: 10.1093/ndt/gfr479. [DOI] [PubMed] [Google Scholar]
- 40.Apostolov EO, Ray D, Savenka AV, et al. Chronic uremia stimulates LDL carbamylation and atherosclerosis. J Am Soc Nephrol. 2010;21:1852–1857. doi: 10.1681/ASN.2010040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koeth RA, Kalantar-Zadeh K, Wang Z, et al. Protein carbamylation predicts mortality in ESRD. J Am Soc Nephrol. 2013;24:853–861. doi: 10.1681/ASN.2012030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Apolito M, Du X, Zong H, et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J Clin Invest. 2010;120:203–213. doi: 10.1172/JCI37672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel KP, Luo FJ, Plummer NS, et al. The production of p-cresol sulfate and indoxyl sulfate in vegetarians versus omnivores. Clin J Am Soc Nephrol. 2012;7:982–988. doi: 10.2215/CJN.12491211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopple JD. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am J Kidney Dis. 2001;37(1 Suppl 2):S66–S70. doi: 10.1053/ajkd.2001.20748. [DOI] [PubMed] [Google Scholar]
- 45.Bellasi A, Mandreoli M, Baldrati L, et al. Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol. 2011;6:883–891. doi: 10.2215/CJN.07810910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ix JH, Chonchol M, Laughlin GA, et al. Relation of sex and estrogen therapy to serum fibroblast growth factor 23, serum phosphorus, and urine phosphorus: the Heart and Soul Study. Am J Kidney Dis. 2011;58:737–745. doi: 10.1053/j.ajkd.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cirillo M, Ciacci C, De Santo NG. Age, renal tubular phosphate reabsorption, and serum phosphate levels in adults. N Engl J Med. 2008;359:864–866. doi: 10.1056/NEJMc0800696. [DOI] [PubMed] [Google Scholar]
- 48.Fouillet H, Barbara J, Bos C, et al. Urea-nitrogen production and salvage are modulated by protein intake in fed humans: results of an oral stable-isotope-tracer protocol and compartmental modeling. Am J Clin Nutr. 2008;87:1702–1714. doi: 10.1093/ajcn/87.6.1702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.