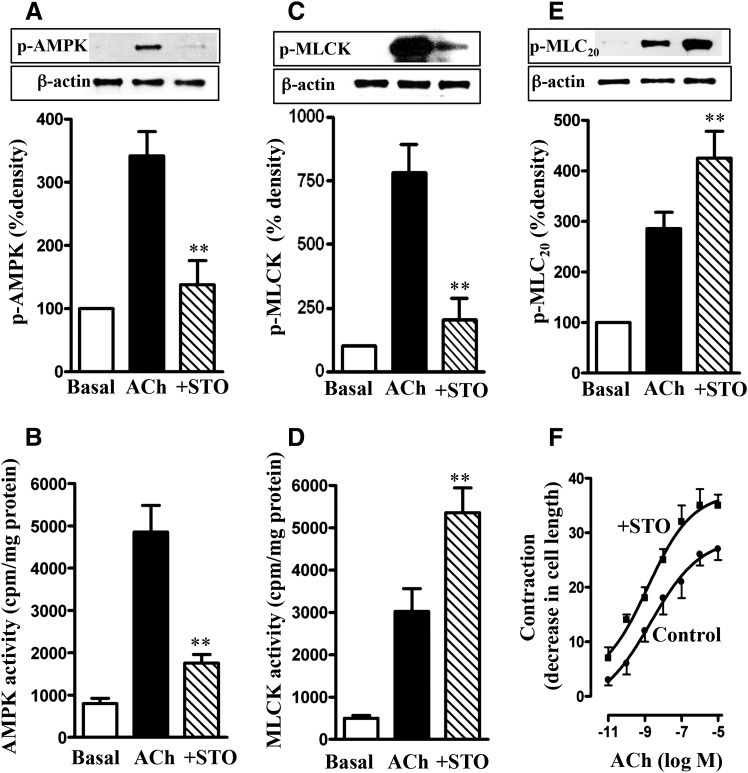
Fig. 1.
Regulation of MLCK activity and muscle contraction by AMPK. Longitudinal muscle cells isolated from control mice were treated with 1 µM ACh in the presence or absence of CaMKK inhibitor STO609 (STO; 10 µM) for 30 seconds. Cells were preincubated with STO609 for 15 minutes before the addition of ACh for 30 seconds. (A) Phosphorylation of AMPK at Thr172 by CaMKK. Phosphorylation of AMPK was analyzed by Western blot using phospho-specific antibody. (B) Stimulation of ACh-induced AMPK activity by CaMKK. AMPK activity was measured by immunokinase assay using MLCK as substrate and [32P]ATP. The results are expressed as cpm/mg protein. (C) Phosphorylation of MLCK by AMPK. Phosphorylation of MLCK was measured in cells labeled with 32P. (D) Feedback inhibition of ACh-induced MLCK activity by AMPK. MLCK activity was measured by immunokinase assay using MLC20 as substrate and [32P]ATP, and the results are expressed as cpm/mg protein. (E) Attenuation of ACh-induced MLC20 phosphorylation by AMPK. MLC20 phosphorylation at Ser19 was measured by immunoblot using phospho-specific antibody. (F) Attenuation of ACh-induced muscle contraction by AMPK. Muscle contraction was measured by scanning micrometry and is expressed as the percentage of decrease in cell length from the basal length (108 ± 7 µm). Values are means ± S.E.M. of four experiments. **P < 0.05, significantly different from response to ACh alone.