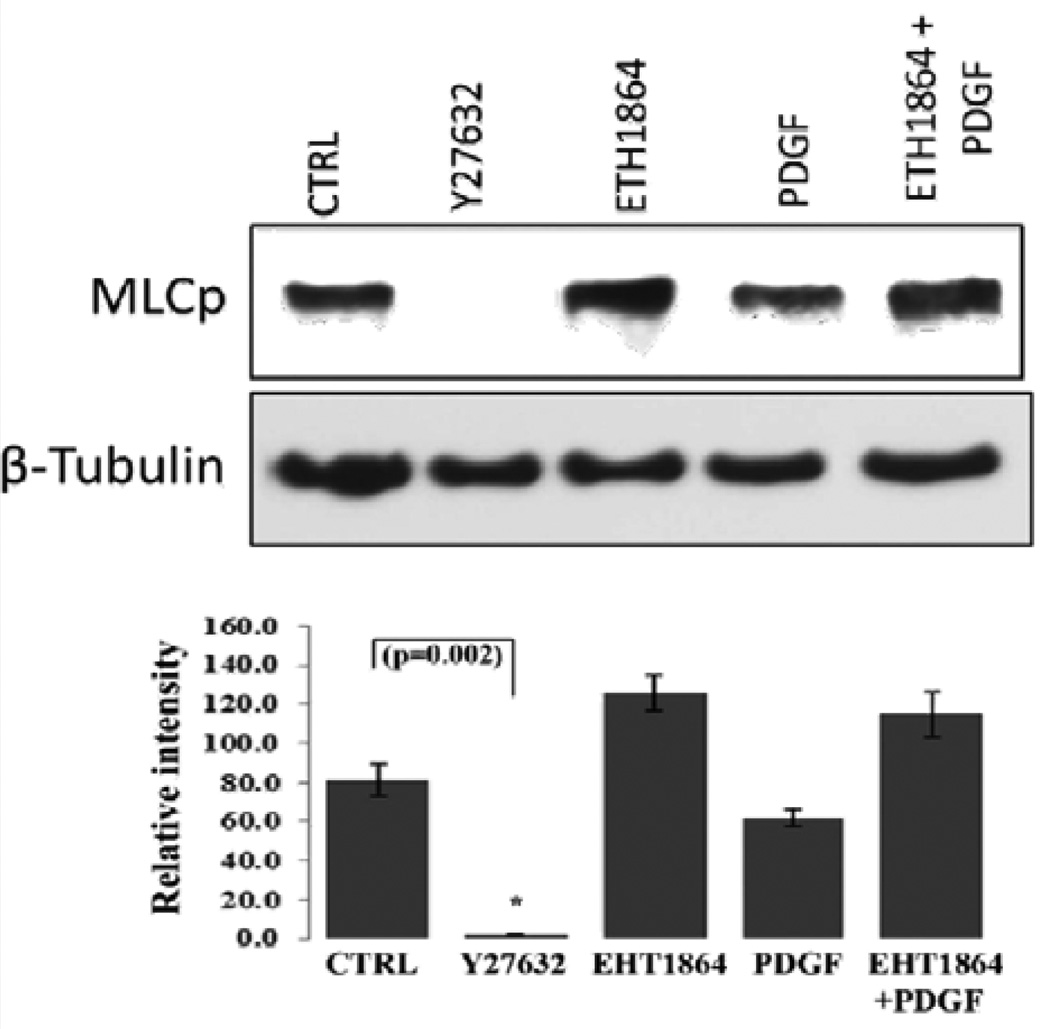
Figure 5.
Effects of PDGF and Rac GTPase inhibitor on HTM cell myosin light chain phosphorylation. Confluent cultures of HTM cells were maintained in 1% FBS overnight and treated with the Rac GTPase inhibitor- EHT1864 (20 µM) or Rho kinase inhibitor (Y27632, 5 µM). A second set of cells under serum free conditions was treated with PDGF (10 ng/ml) for 4 h. Rac GTPase inhibitor and PDGF exerted contrasting but moderate effects on MLC phosphorylation, with Rac inhibitor activating and PDGF inhibiting the response, compared to the Rho kinase inhibitor, which caused a complete suppression of MLC phosphorylation (P<0.05; n=3). Activation of Rac GTPase by PDGF (10 ng/ml for 2 h, in serum free media) after pretreatment with the Rac inhibitor EHT1864 (20 µM in 1% serum media for 4 h) did not cause any additional changes in the levels of myosin light chain phosphorylation indicating that PDGF induced changes in MLC phosphorylation appear to be mediated through the Rac GTPase. β-Tubulin immunoblot was used for loading control. Histograms show quantitative changes in MLC phosphorylation based on densitometric analysis using Image J. Error bars represent standard error. N=3.