Fig. 1.
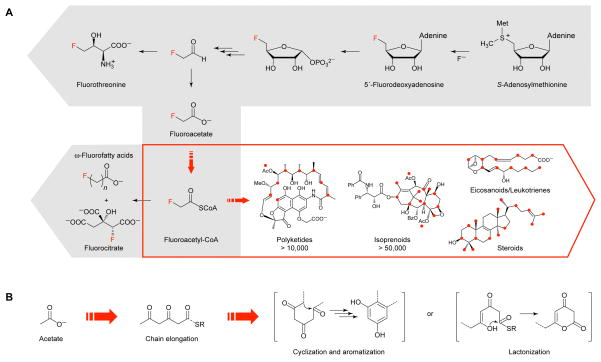
Synthetic biology of fluorine. (A) The fluoroacetate pathway and its metabolites represent the known scope of biological fluorine chemistry, starting with fluoride and S-adenosylmethionine, to produce fluoroacetate and fluorothreonine as the end products (right to left, grey box). This scope could be greatly expanded by engineering downstream pathways to use fluoroacetate as a building block for introduction of fluorine site-selectively into large families of natural products constructed from acetate backbones (left to right, red box). Red dots represent positions that could in principle be fluorinated by incorporation of a fluoroacetate monomer without altering the carbon skeleton, including locations where fluorine would replace a methyl group derived from propionate or where downstream tailoring steps have occurred on the final structure. (B) Assembly of acetate units in the biosynthesis of polyketide natural products.