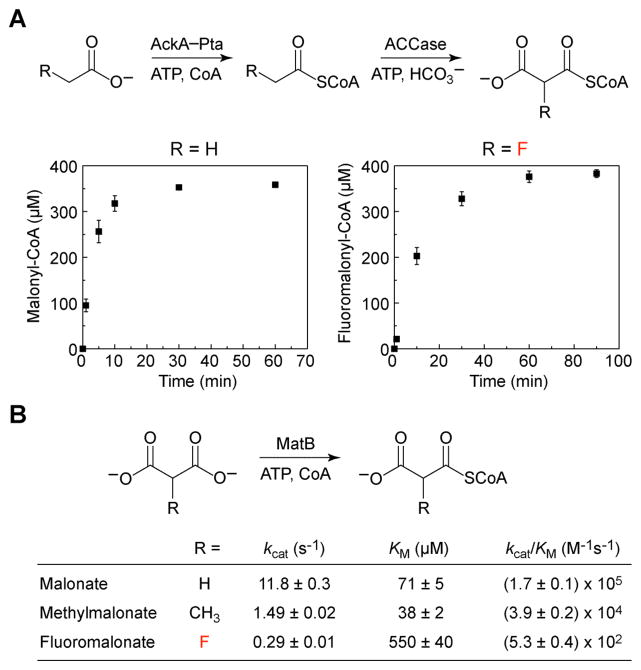
Fig. 2.
Enzymatic production of activated extender units for C–C bond formation reactions. (A) Formation of malonyl-CoA (left) and fluoromalonyl-CoA (right) from 500 μM CoA and either acetate or fluoroacetate, respectively (AckA, acetate kinase; Pta, phosphotransacetylase; ACCase, acetyl-CoA carboxylase). Values are reported as the mean ± s.d. (n = 3). (B) Kinetic parameters for malonate activation (MatB, malonyl-CoA synthetase). Kinetic parameters are reported as mean ± s.e. (n = 3) as determined from non-linear curve-fitting. Error in the kcat/KM parameter was obtained from propagation of error from the individual kinetic terms.