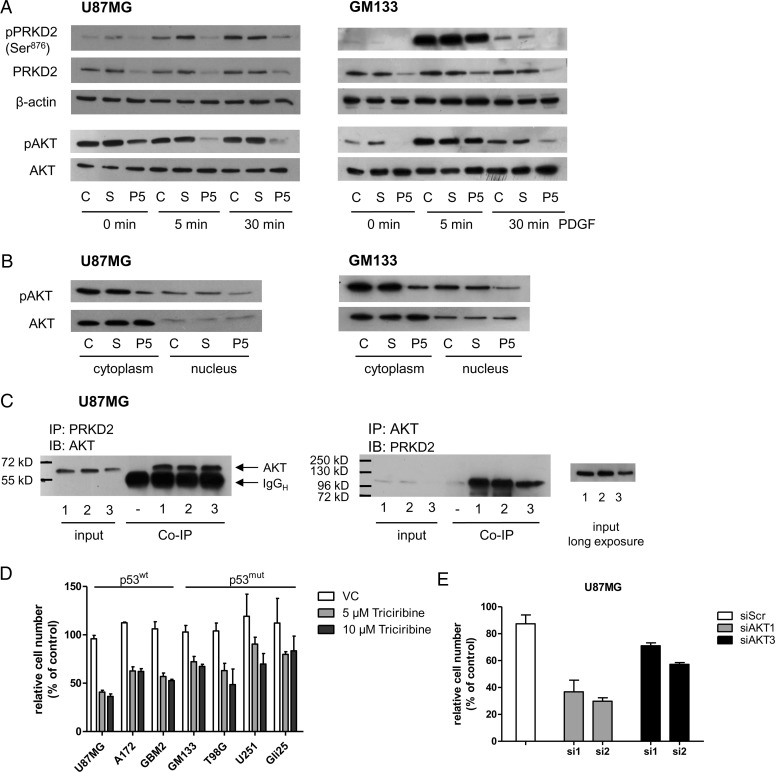
Fig. 3.
PRKD2 modulates AKT activity in glioma cells. (A) Three days post silencing, U87MG and GM133 were stimulated with 10 ng/ml PDGF for 5 and 30 minutes. Total cell lysates were immunoblotted, and activation of indicated proteins was monitored using phospho-specific antibodies. To ensure comparable loading of lysates, the corresponding pan-proteins or actin were used as controls. One representative immunoblot (of at least 3 independent experiments) is shown. AKT antibodies used recognize AKT isoforms 1, 2, and 3. (B) The effect of PRKD2 silencing on AKT phosphorylation was studied in cytosolic and nuclear fractions by Western blotting. C = untreated cells, S = siScr, P5 = siP5. (C) Co-immunoprecipitation experiments indicating physical interaction of PRKD2 and AKT. Protein extracts of U87MG cells (3 independent dishes, ‘1, 2, 3′) were immunoprecipitated with the indicated antibodies, followed by immunoblotting. The association of proteins was confirmed by reverse immunoprecipitation. Input levels were detected to ensure comparable loading. Sham precipitation (“-”) was performed to exclude unspecific immunoreactions. (D) The indicated cell lines were incubated in the presence of 5 and 10 µM triciribine. Ethanol was used as vehicle control. Five days after addition of triciribine/ethanol, cells were harvested and counted. Results represent mean ± SD (n = 4) of relative cell numbers normalized to untreated cells. (E) Two different siRNA constructs (si1; si2) were used to silence AKT1 and AKT3 expression. Untreated cells and cells transfected with nontargeting siRNA (siScr) were used as controls. Cells were harvested and counted on day 6 post silencing. Results represent mean ± SD of relative cell numbers normalized to untreated cells from one representative experiment done in triplicates.