Figure 3. Importance of UGT1A genotypes in raloxifene glucuronide formation in human tissues and in plasma samples from raloxifene-treated subjects.
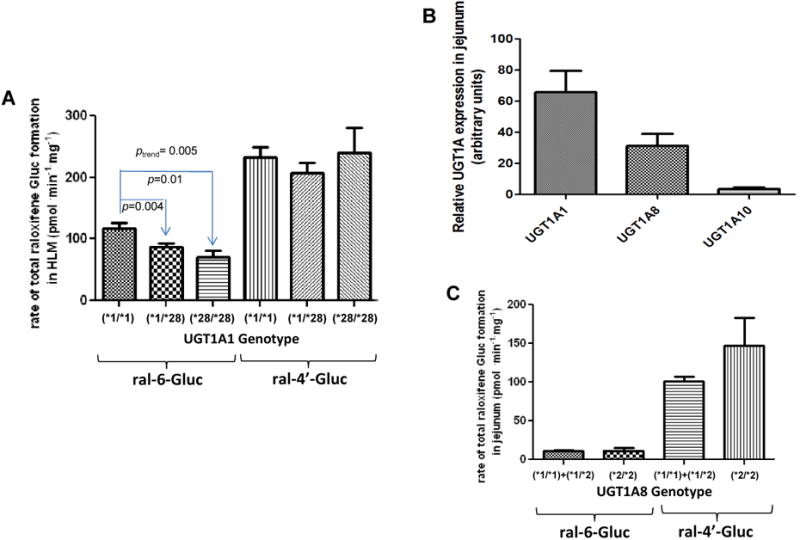
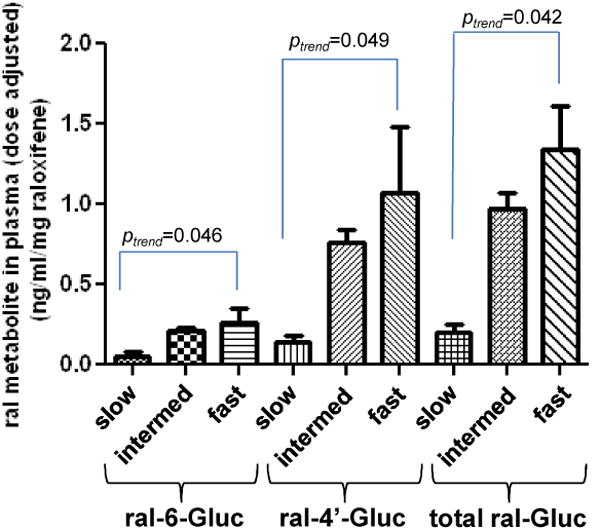
Glucuronidation activity assays were performed by incubation of raloxifene with HLM or HJH, and raloxifene glucuronides were analyzed by UPLC or UPLC/MS/MS as described in Materials and methods. The relative abundance of UGTs 1A1, 1A8 and 1A10 mRNAs in the jejunum was measured in 5 individual jejunum specimens by qPCR using the ΔΔCt method. (A) Rate of raloxifene glucuronide formation in HLM stratified by UGT1A1 genotype; (B) Relative expression levels of UGT1A mRNA in jejunum; (C) Rate of total raloxifene glucuronide formation in HJH stratified by UGT1A8 genotype; (D) Levels of raloxifene glucuronides in plasma stratified by UGT1A8 genotype. Subjects with the UGT1A8 (*1/*3) genotype were defined as slow raloxifene metabolizers (slow), subjects with either the UGT1A8 (*1/*1) or UGT1A8 (*1/*2) genotypes were defined as intermediate raloxifene metabolizers (intermed), and subjects with the UGT1A8 (*2/*2) genotype were defined as fast raloxifene metabolizers (fast). The Student's t-test was used to compare raloxifene Gluc formation in HLM from subjects with UGT1A1 (*1/*28) or (*28/*28) genotypes with the wild type UGT1A1 (*1/*1), and to compare total raloxifene glucuronide formation in HJH from subjects with UGT1A8 (*2/*2) genotype with UGT1A8 (*1/*1+*1/*2) genotypes. The one-way ANOVA trend test was used to examine the overall effect of UGT1A1 genotype on rate of ral-6-Gluc and ral-4′-Gluc formation in HLM. The Jonckheere-Terpstra trend test was used to examine the overall effect of UGT1A8 genotype on ral-6-Gluc, ral-4′-Gluc and total raloxifene glucuronide levels in plasma from women treated with raloxifene. Samples from subjects treated by 60 mg and 30 mg raloxifene daily were combined after plasma raloxifene and glucuronide levels were adjusted for raloxifene dose (mg) for each subject.
