Scheme 1.
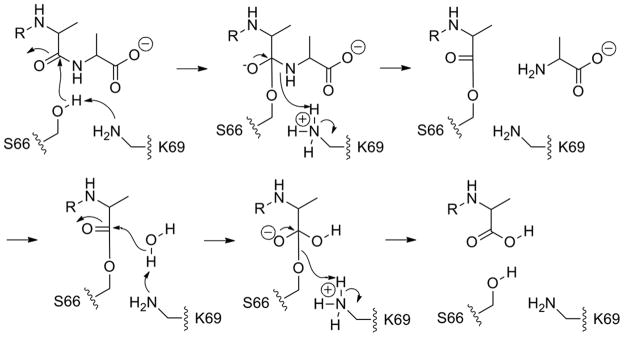
Proton-transfer events in the catalytic event. As Lys69 activates Ser66, it shuttles the proton to the nitrogen of the departing terminal D-Ala, whereby it reverts back to the free-base state. The free-base Lys69 now promotes a water molecule for the deacylation step; hence PBP 6 enjoys symmetry in catalysis. The proton from the water molecule is shuttled to the departing serine Oγ, returning Lys69 to its ground-state free-base form.
