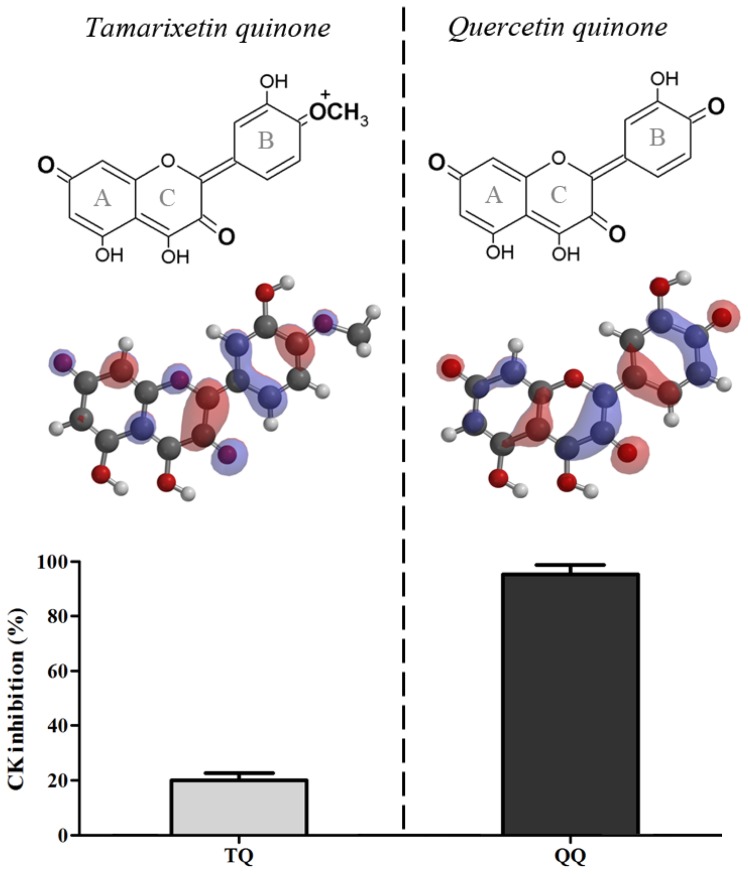
Figure 1.
Structure and Lowest Unoccupied Molecular Orbital (LUMO) localization map of the preferred tautomer of quercetin quinone and tamarixetin quinone, and the effect of quercetin and tamarixetin oxidation on the enzyme activity of creatine kinase (CK). The carbonyl groups of quercetin quinone are positioned at maximal distance within the molecule and the LUMO is distributed over the phenolic rings, which explains why it behaves as a soft electrophile. Tamarixetin quinone has a positive charge and the LUMO is focused in the B-ring, which makes it a relatively hard electrophile. Quercetin and tamarixetin (50 μM) were oxidized by 50 μM H2O2 and 0.4 or 3.2 nM horseradish peroxidase (HRP), respectively, to obtain an equal rate of oxidation (5 μM/min). In the presence of 6.2 μM CK, the enzyme activity of CK was measured. Data are shown as mean ± SE (n = 4).