Abstract
Electrical stimulation of the brain has a 2000 year history. Deep brain stimulation (DBS), one form of neurostimulation, is a functional neurosurgical approach in which a high‐frequency electrical current stimulates targeted brain structures for therapeutic benefit. It is an effective treatment for certain neuropathologic movement disorders and an emerging therapy for psychiatric conditions and epilepsy. Its translational journey did not follow the typical bench‐to‐bedside path, but rather reversed the process. The shift from ancient and medieval folkloric remedy to accepted medical practice began with independent discoveries about electricity during the 19th century and was fostered by technological advances of the 20th. In this paper, we review that journey and discuss how the quest to expand its applications and improve outcomes is taking DBS from the bedside back to the bench.
Keywords: neurostimulation, electrical stimulation, deep brain stimulation
What is Deep Brain Stimulation?
Deep brain stimulation (DBS), a type of neuromodulation, is a restorative functional neurosurgical approach that has been established as an effective therapy for essential tremor,1 dystonia,2 and the movement disorders associated with Parkinson's disease (PD).3 DBS is also an emerging therapy for depression,4, 5, 6 obsessive‐compulsive disorder (OCD),7, 8 and epilepsy.9, 10, 11 More than 80,00012 people have been successfully implanted with DBS devices worldwide; this number is expected to grow dramatically.
Current DBS systems use a continuous, high frequency (100–250 Hz) pulse train applied to a surgically implanted stimulating electrode.13 The procedure involves obtaining stereotactic coordinates for the target structure from software that merges magnetic resonance imaging (MRI) of the patient's brain with a brain atlas. During surgery, microelectrode recording verifies a trajectory to the target using region‐specific neural activity as functional landmarks.14 Once verified, the microelectrode is withdrawn, a stimulating electrode implanted, secured to the skull, and connected subcutaneously to a pulse generator implanted in the chest area (Figure 1). After recovery, stimulation is initiated, and optimal stimulation parameters (amplitude, frequency, pulse width, and active contact) are empirically determined.
Figure 1.
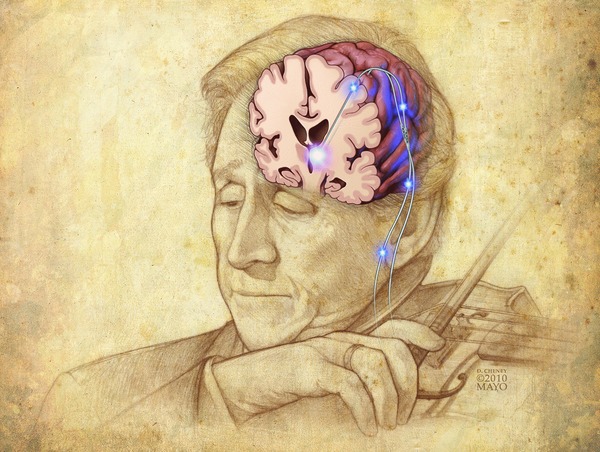
Illustration of deep brain stimulation electrodes. Illustration appears courtesy of David Cheney.
DBS is only one form of neuromodulation. Others include transcranial magnetic stimulation, electroconvulsive therapy, and motor cortex stimulation. Electrical stimulation was also used in early experiments aimed at localizing brain function and continues to be used in neurosurgical procedures requiring brain mapping. DBS differs from these in that the target structures are deep within the brain, such as the thalamus and globus pallidus. DBS, as we know it today, grew out of stereotactic functional neurosurgery aimed at the ablation of targeted areas thought to contribute to the pathology of dyskinesia and tremor in PD. Here we review the translational journey of DBS from anecdotal remedy to accepted therapeutic tool and discuss some remaining obstacles to its application.
Translating Scientific Discoveries and Technological Innovations into Treatment
Electrical stimulation of the nervous system for therapeutic benefit has a long history. In 46 A.D. Scribonius Largus, a Roman physician, recommended applying electric fish to patients' heads in order to relieve headaches.15, 16, 17 This practice for headaches and other maladies, such as hemorrhoids, gout, and epilepsy, continued up to the 1700s. It was not until the late 1900s, however, that the pathway for translation of DBS from bench to bedside truly began. See Figure 2 for a timeline highlighting major events in DBS' path of translation.
Figure 2.
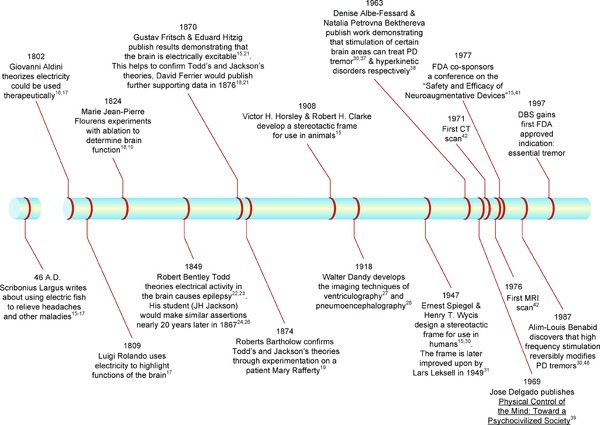
Timeline highlighting major events in the pathway of translation of deep brain stimulation (DBS). PD, Parkinson's disease; CT, computed tomography.
Early investigations into brain function
Early investigations into the localization of function in the brain employed either electrical stimulation or ablation. Pioneers in this work include Luigi Rolando,17 Marie Jean‐Pierre Flourens,18, 19 Giovanni Aldini,16, 17, 20 Gustav Fritsch and Eduard Hitzig,15, 16, 17, 18, 19, 21 and David Ferrier.17, 18, 19, 21 In 1809, Rolando utilized electrical current to stimulate the brains of animals, uncovering the functionality of certain parts of the brain.17 In contrast, Flourens developed ablation as a technique to define brain function.18 Flourens was opposed to theories of cerebral localization and believed that the cerebrum functioned as a whole. He published his landmark work in 1824, in which he concluded that the cerebral hemispheres were not excitable and that control of motor function lay with the brainstem.18, 19 Aldini, after early experiments into the effects of electrical current on brain regions, theorized that electrical current may also have therapeutic potential.16, 17 He began to validate his theory using cadavers and subsequently used electrical current to treat patients with various mental disorders. In 1802, Aldini publicly took the bodies of three recently deceased criminals and proceeded to stimulate both the body and brain of these criminals with electricity, producing muscular contractions.16 He observed that stimulating one side of the brain caused muscles to contract on the opposite side of the face. This discovery would go unheeded for nearly 70 years.16, 20
Mapping brain function and linking electrical activity in the brain to disease
Fritsch and Hitzig observed contractions similar to those observed by Aldini while treating soldiers during the Danish‐Prussian war of 1863.15, 21 They began to experiment on dogs to explore and expand on these observations using electrical current to stimulate their cortexes, triggering muscular contractions. In 1870, they published the results of these experiments, including the observation that increasing the intensity of the current could increase the intensity of the response. They were the first to provide evidence that the cerebral cortex was involved in motor function, that function was localized within the cerebral cortex, and that the cortex was electrically excitable. David Ferrier expanded on these findings through the use of both ablation and electrical stimulation.18, 21 He published The Functions of the Brain in 1876 in which he mapped the functions of sensory and motor areas across several species. Both Ferrier's and Fritsch/Hitzig's experiments were influenced by and helped to confirm the earlier clinical observations and hypotheses made previously by Robert Bentley Todd22, 23 and later by John Hughlings Jackson.21, 24, 25, 26 Todd hypothesized that epilepsy “…shows itself in the unnatural development of nervous force…which…may be compared to the electrical phenomenon described by Faraday….”22 Jackson, a student of Todd's, studied patients with epilepsy and proposed that the convulsions he saw in epileptic patients were the result of a “sudden disorderly expenditure of force.”24, 26 His idea was formalized in his 1873 definition of epilepsy as “the name for occasional, sudden, excessive, rapid, and local discharge of grey matter.”25 This was a milestone in that it linked electrical activity in the brain to disease.
From animal models to patients: a question of ethics
While the work of Fritsch, Hitzig, and Ferrier helped to confirm the suspicions of Todd and Jackson, none of their experiments were conducted in humans. This changed in 1874, when Roberts Bartholow began a series of experiments on Mary Rafferty, a 30‐year‐old cancer patient.19 The cancer and an associated infection caused a large area of her skull to erode, exposing the underlying brain tissue. Realizing that there was little that could be done to cure the patient, Bartholow sought Rafferty's permission to perform a series of experiments in which he electrically stimulated parts of her exposed brain. He found that mechanical stimulation of the brain did not result in a reaction, whereas electrical stimulation caused a variety of muscular contractions and eventually seizures. In causing a seizure in Rafferty, he confirmed prior suspicions about the origin of seizures. However, Bartholow's experiments on Rafferty did not go unnoticed by the medical community.19 There was a great amount of opposition towards this experimentation, resulting in the American Medical Association's condemnation of his actions and proposition of a resolution stating that “no member of the medical profession is justified in experimenting upon his patient, except for the purpose…of saving said patient's life.”19 In addition to the proposed resolution, the British Medical Journal published a blistering critique of Bartholow's experiment. Bartholow responded with an apology in which he stated that “I now know that I was mistaken” and “to repeat such experiment with the knowledge we have now…would be… criminal.” Despite the objections of the medical community, it was recognized that his results did have scientific merit, and on this ground they were praised by Ferrier himself.19
Lost in translation
In 1882, Ezio Sciamanna experimented on Ferdinando Rinalducci, a patient with a traumatic brain injury.19, 20 He electrically stimulated various parts of Rinalducci's exposed brain tissue and elicited various muscular contractions based on the site and intensity of stimulation. The patient died after four days of experimentation, however, unlike Bartholow's experiments, there was little international backlash, possibly because the report was published in Italian in a little known journal.19 The following year, Alberto Alberti began conducting his experiments on Severo Velo, an epileptic.20 These experiments would continue for 8 months, during which time Alberti would, like those before him, stimulate the patient's brain with electric current and observe the resulting muscular contractions.
Ethical treatment revisited
Nearly 20 years after Bartholow's work was published, William Ransom used electrical stimulation on a patient whose cortex had been exposed due to trephination.19 He observed similar results as his predecessors. However, like Sciamanna and Alberti, Ransom did not face the same backlash as Bartholow. Ransom's patient presumably had normal intelligence, whereas Bartholow's patient was reported to have mental deficits; therefore, Ransom's patient was more capable of giving informed consent. Furthermore, Ransom's patient was studied for several months with no reported serious side effects, whereas Bartholow's died after a few days. Despite the lack of objection from Ransom's studies, further developments advancing DBS would not occur for another 16 years.
The dawn of stereotaxis and brain imaging
In 1908 Victor Horsley and Robert H. Clarke developed the first stereotactic frame for use in animals,15 which was crucial for the precise placement of electrodes. However, the original system was developed for small to medium sized animals and relied upon the use of coordinates and a brain atlas. Additionally, as a result of individual heterogeneity, it was not very accurate. This was partially mitigated in 1918 when Walter Dandy developed x‐ray ventriculography27 and pneumoencephalography.28 With these techniques, neurosurgeons could visualize the brain for the first time. Despite the advantages these techniques had, they were still poorly tolerated by patients.
Pioneering the therapeutic use of electrical stimulation of the brain
With these visualization techniques, neurosurgeons were able to further explore the potential of electrical stimulation of the brain. In 1937, Wilder Penfield published the results of a series of 163 operations during which electrical stimulation was performed. Penfield was careful to state that “stimulation was only carried out when there was therapeutic justification for it.”29 Penfield used electrical stimulation to help him localize the origin of seizures in epileptic patients and to help him map out the sensory and motor functions of various areas within the cortex of the brain.
Despite advances in imaging technology and brain mapping, it was not until 1947 that Ernest Spiegel and Henry T. Wycis designed a stereotactic frame for use in humans,15, 30 which was later improved upon by Lars Leksell in 1949.31 This ushered in a new era in neurosurgery; one that would eventually lead to the development of DBS.
In the following years, there were many advances in the field of electrical stimulation. Denise Albe‐Fessard pioneered the use of microelectrodes to obtain intracellular recordings of mammalian brains.30, 32, 33 In 1955, she reported on intracellular recordings obtained from the cerebral cortex of a cat; later, she reported on microelectrode recording in the human brain as a means to localize surgery for conditions like Parkinson's and dyskinesias.34, 35, 36 Additionally, she observed that stimulation of the ventrointermediate nucleus of the thalamus at 100–200 Hz could inhibit tremor in patients with PD.30, 37 That same year Natalia Petrovna Bekthereva published her groundbreaking work on chronic stimulation for the treatment of hyperkinetic disorders38—the first report of DBS for movement disorders.17 Unfortunately, her work, published in Russian, was not well known at the time due to limited readership.
Early use of DBS in psychiatry
It was also in 1963 that Jose Delgado implanted an electrode in the brain of a bull, and in a famously theatrical display, used a radio signal to stop the bull from charging.17 Later, in 1969, he published Physical Control of the Mind: Toward a Psychocivilized Society,39 in which he discussed techniques to implant intracranial electrodes in humans, their therapeutic and diagnostic use in psychological disorders, and the ethical implications of his work. Carl Wilhelm Sem‐Jacobsen, another pioneer in the field of DBS, in 1963 published a paper describing the effect of electrical stimulation in psychiatric patients.30 He found that electrical stimulation of areas of the frontal lobe resulted in a reduction in symptoms that were either temporary or resulted in a “complete freedom from symptoms.” He would later report on the effects of electrical stimulation in patients with PD.30 Of note, Sem‐Jacobsen did not consider stimulation as a treatment but as a means to evaluate brain targets prior to lesioning.
DBS: an efficacious treatment versus placebo?
In contrast to Sem‐Jacobsen's approach, Irving Cooper experimented with DBS as a treatment for a variety of conditions including cerebral palsy, epilepsy, and spasticity.15, 40 In 1977, the FDA co‐sponsored a conference on the “Safety and Efficacy of Neuroaugmentative Devices.”15, 41 It was concluded that, for most indications, the FDA felt there was not sufficient evidence of efficacy and safety.41 In response, Cooper performed a double blinded trial of cerebellar stimulation for cerebral palsy, only to find that it was not as efficacious as originally believed and a large placebo effect was present.15
Advances in imaging: CT and MRI
Despite Cooper's setback, the 1970s were important decade for the development of DBS‐related technologies. In 1971, the first computerized tomography (CT) scan was performed;42 its success led to its widespread use by 1980.43 A few years later, MRI scans were first utilized and their efficacy tested.42, 44, 45 The chief advantages of CT scans and MRIs over previous technologies were that they were noninvasive and provided higher resolution.46 Today, preplanning with MRI is common as it allows entry point and trajectory planning and intraoperative use.47
Advent of modern DBS
In 1987, Alim‐Louis Benabid and colleagues made a breakthrough discovery which helped to bring DBS to where it stands today. In patients with PD, they found that DBS at frequencies higher than 100 Hz reversibly modified patients' tremors, safely mimicking lesioning.30, 48 This discovery reinvigorated research in the area of DBS, leading to the development and approval of the DBS systems available today.
Barriers to translation
DBS has proven to be a useful therapeutic option for many patients. However, several factors influenced, and continue to influence, the translational process. These factors include the need for technological and scientific discoveries/developments, economic and regulatory barriers, and ethical issues surrounding DBS. In order for DBS to progress, there needed to be several technological and scientific discoveries/developments. While significant progress has been made, a lack of a clear understanding of the mechanism of DBS, identification of the most appropriate targets and patients for treatment, and improving the devices and procedures in order to reduce complications all remain challenges to further development of this technology.49, 50 The translation of DBS is further complicated by regulatory, social, and economic barriers. Investigators undertaking studies of DBS using existing devices require an Investigational Device Exemption from the FDA. This warrants data on the device, which is available only from the manufacturer, creating an additional barrier for investigators.51 Additionally, there is a potential for resource underuse due to restrictive intellectual property rights held by manufacturers.52
Translating DBS to the clinic is difficult not only due to the high monetary cost, but also because of the emotional burden of the procedure and maintenance of the device. This barrier can be exacerbated due to a media bias which tends to overstate the benefits of the procedure and downplay the risks,53 leading to false expectations for the public and scientific community. In order for DBS to progress there needs to be a concerted effort to accurately portray the risks, benefits, efficacy and cost‐effectiveness54, 55, 56 of DBS; furthermore, additional efforts to make these procedures accessible to all eligible patients are needed.
Acknowledgments
Thanks to David Cheney, Colleen Crane, Penelope S. Duffy Ph.D., and Greg Molnar Ph.D. for their valuable comments and contributions. This publication was supported by NIH/NCATS CTSA Grant Number TL1 RR0024152 (MRG/AJG) as well as NIH/NINDS Grant Numbers K08 NS 52252, R01 NS 70872, and R01 NS 74013 (KHL). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1. Benabid AL, Pollak P, Seigneuret E, Hoffmann D, Gay E, Perret J. Chronic VIM thalamic stimulation in Parkinson's disease, essential tremor and extra‐pyramidal dyskinesias. Acta Neurochir Suppl (Wien). 1993; 58: 39–44. [DOI] [PubMed] [Google Scholar]
- 2. Greene P. Deep‐brain stimulation for generalized dystonia. N Engl J Med. Feb 3 2005; 352(5): 498–500. [DOI] [PubMed] [Google Scholar]
- 3. Deuschl G, Schade‐Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, Daniels C, Deutschlander A, Dillmann U, Eisner W, et al. A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med. Aug 31 2006; 355(9): 896–908. [DOI] [PubMed] [Google Scholar]
- 4. Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment‐resistant depression. Neuron. Mar 3 2005; 45(5): 651–660. [DOI] [PubMed] [Google Scholar]
- 5. Hardesty DE, Sackeim HA. Deep brain stimulation in movement and psychiatric disorders. Biol Psychiatry. Apr 1 2007; 61(7): 831–835. [DOI] [PubMed] [Google Scholar]
- 6. Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. Jan 2008; 33(2): 368–377. [DOI] [PubMed] [Google Scholar]
- 7. Greenberg BD, Malone DA, Friehs GM, Van Dycke A, Goethals M, Goossens L, Van Zandijcke M, De Smedt, Dewaele I, Achten R, Wadman W, et al. Three‐year outcomes in deep brain stimulation for highly resistant obsessive‐compulsive disorder. Neuropsychopharmacology. Nov 2006; 31(11): 2384–2393. [DOI] [PubMed] [Google Scholar]
- 8. Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment‐refractory obsessive‐compulsive disorder: the search for a valid target. Neurosurgery. Jul 2007; 61(1): 1–11; discussion 11–13. [DOI] [PubMed] [Google Scholar]
- 9. Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. Jun 2002; 43(6): 603–608. [DOI] [PubMed] [Google Scholar]
- 10. Boon P, Vonck K, De Herdt V, Van Dycke A, Goethals M, Goossens L, Van Zandijcke M, De Smedt, Dewaele I, Achten R, et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. Aug 2007; 48(8): 1551–1560. [DOI] [PubMed] [Google Scholar]
- 11. Vonck K, Boon P, Van Roost D. Anatomical and physiological basis and mechanism of action of neurostimulation for epilepsy. Acta Neurochir Suppl. 2007; 97(Pt 2): 321–328. [DOI] [PubMed] [Google Scholar]
- 12. Medtronic . About DBS Therapy. 2012; http://www.medtronic.com/patients/parkinsons‐disease/therapy/index.htm. Accessed 1 May 2012. [Google Scholar]
- 13. McIntyre CC, Butson CR, Maks CB, Noecker AM. Optimizing deep brain stimulation parameter selection with detailed models of the electrode‐tissue interface. Conf Proc IEEE Eng Med Biol Soc. 2006; 1: 893–895. [DOI] [PubMed] [Google Scholar]
- 14. Priori A, Egidi M, Pesenti A, Rohr M, Rampini P, Locatelli M, Tamma F, Caputo E, Chiesa V, Barbieri S. Do intraoperative microrecordings improve subthalamic nucleus targeting in stereotactic neurosurgery for Parkinson's disease? J Neurosurg Sci. Mar 2003; 47(1): 56–60. [PubMed] [Google Scholar]
- 15. Schwalb JM, Hamani C. The history and future of deep brain stimulation. Neurotherapeutics. Jan 2008; 5(1): 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parent A. Giovanni Aldini: from animal electricity to human brain stimulation. Can J Neurol Sci. Nov 2004; 31(4): 576–584. [DOI] [PubMed] [Google Scholar]
- 17. Sironi VA. Origin and evolution of deep brain stimulation. Front Integr Neurosci. 2011; 5: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pearce JM. Marie‐Jean‐Pierre Flourens (1794–1867) and cortical localization. Eur Neurol. 2009; 61(5): 311–314. [DOI] [PubMed] [Google Scholar]
- 19. Morgan JP. The first reported case of electrical stimulation of the human brain. J Hist Med Allied Sci. Jan 1982; 37(1): 51–64. [DOI] [PubMed] [Google Scholar]
- 20. Zago S, Ferrucci R, Fregni F, Priori A. Bartholow, Sciamanna, Alberti: pioneers in the electrical stimulation of the exposed human cerebral cortex. Neuroscientist. Oct 2008; 14(5): 521–528. [DOI] [PubMed] [Google Scholar]
- 21. Gross CG. The discovery of motor cortex and its background. J Hist Neurosci. Jul–Sep 2007; 16(3): 320–331. [DOI] [PubMed] [Google Scholar]
- 22. Todd RB. The Lumleian Lectures for 1849. On the pathology and treatment of convulsive diseases. Epilepsia. Jul 2005; 46(7): 995–1009. [DOI] [PubMed] [Google Scholar]
- 23. Reynolds EH. Todd, Faraday, and the electrical basis of epilepsy. Epilepsia. Aug 2004; 45(8): 985–992. [DOI] [PubMed] [Google Scholar]
- 24. Jackson JH. Note on Regional Palsy and Spasm. Br Med J. Dec 28 1867; 2(365): 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson JH. On the anatomical, physiological, and pathological investigations of epilepsies. West Riding Lunatic Asylum Med Rep. 1873; 3: 315–349. [Google Scholar]
- 26. York GK, 3rd , Steinberg DA. Hughlings Jackson's neurological ideas. Brain. Oct 2011; 134(Pt 10): 3106–3113. [DOI] [PubMed] [Google Scholar]
- 27. Dandy WE. Ventriculography following the injection of air into the cerebral ventricles. Ann Surg. Jul 1918; 68(1): 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dandy WE. Rontgenography of the brain after the injection of air into the spinal canal. Ann Surg. Oct 1919; 70(4): 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. December 1, 1937; 60(4): 389–443. [Google Scholar]
- 30. Hariz MI, Blomstedt P, Zrinzo L. Deep brain stimulation between 1947 and 1987: the untold story. Neurosurg Focus. Aug 2010; 29(2): E1. [DOI] [PubMed] [Google Scholar]
- 31. Leksell L. A stereotaxic apparatus for intracerebral surgery. Acta Chir Scand. 1949; 99: 229–233. [Google Scholar]
- 32. Squire LR, Society for Neuroscience The History of Neuroscience in Autobiography. Washington, DC: Society for Neuroscience; 1996. [Google Scholar]
- 33. Shepherd GM. Creating Modern Neuroscience : The Revolutionary 1950s. Oxford, New York: Oxford University Press; 2010. [Google Scholar]
- 34. Albe‐Fessard D, Buser P. [Intracellular activities collected in the sigmoid cortex of the cat; participation of the pyramidal neurons in the somesthetic evoked potential]. J Physiol (Paris). 1955; 47(1): 67–69. [PubMed] [Google Scholar]
- 35. Albe‐Fessard D, Arfel G, Guiot G, Hardy J, Hertzog EA, P. Identification et de´limitation precise de certaines structures sous‐corticales de l'homme par l'electrophysiologie. C R Hebd Seances Acad Sci. 1961; 253(3): 2412–2414. [PubMed] [Google Scholar]
- 36. Guiot G, Hardy J, Albe‐Fessard D, Arfel G, Vourc'h G, Hertzog E, Aleonard P. Délimitation précise des structures sous‐corticales et identification de noyaux thalamiques chez l'homme par l'électrophysiologie stéréotaxique. Minim Invasive Neurosurg. 20.11.2008 1962; 5(01): 1–18. [Google Scholar]
- 37. Albe Fessard D, Arfel G, Guiot G, Derome P, Dela H, Korn H, Hertzog E, Vourch G, Aleonard P. [Characteristic electric activities of some cerebral structures in man]. Ann Chir. Sep 1963; 17: 1185–1214. [PubMed] [Google Scholar]
- 38. Bekhtereva NP, Grachev KV, Orlova AN, Iatsuksl. [Utilization of multiple electrodes implanted in the subcortical structure of the human brain for the treatment of hyperkinesis]. Zh Nevropatol Psikhiatr Im S S Korsakova. 1963; 63: 3–8. [PubMed] [Google Scholar]
- 39. Delgado JMR. Physical Control of the Mind; Toward a Psychocivilized Society. New York: Harper & Row; 1969. [Google Scholar]
- 40. Das K, Benzil DL, Rovit RL, Murali R, Couldwell WT. Irving S. Cooper (1922–1985): a pioneer in functional neurosurgery. J Neurosurg. Nov 1998; 89(5): 865–873. [DOI] [PubMed] [Google Scholar]
- 41. Gildenberg PL. Evolution of neuromodulation. Stereotact Funct Neurosurg. 2005; 83(2–3): 71–79. [DOI] [PubMed] [Google Scholar]
- 42. Hendee WR. Cross sectional medical imaging: a history. Radiographics. Nov 1989; 9(6): 1155–1180. [DOI] [PubMed] [Google Scholar]
- 43. Hounsfield GN. Computed medical imaging. Science. Oct 3 1980; 210(4465): 22–28. [DOI] [PubMed] [Google Scholar]
- 44. Doyle FH, Gore JC, Pennock JM, Bydder GM, Orr JS, Steiner RE, Young IR, Burl M, Clow H, Gilderdale DJ, et al. Imaging of the brain by nuclear magnetic resonance. Lancet. Jul 11 1981; 2(8237): 53–57. [DOI] [PubMed] [Google Scholar]
- 45. Young IR, Hall AS, Pallis CA, Legg NJ, Bydder GM, Steiner RE. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. Nov 14 1981; 2(8255): 1063–1066. [DOI] [PubMed] [Google Scholar]
- 46. Rezai AR, Kopell BH, Gross RE, Vitek JL, Sharan AD, Limousin P, Benabid AL. Deep brain stimulation for Parkinson's disease: surgical issues. Mov Disord. Jun 2006; 21(Suppl 14): S197–218. [DOI] [PubMed] [Google Scholar]
- 47. Huston OO, Watson RE, Bernstein MA, McGee KP, Stead SM, Gorman DA, Lee KH, Huston J. Intraoperative magnetic resonance imaging findings during deep brain stimulation surgery. J Neurosurg. Oct 2011; 115(4): 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schiefer TK, Matsumoto JY, Lee KH. Moving forward: advances in the treatment of movement disorders with deep brain stimulation. Front Integr Neurosci. 2011; 5: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bell E, Mathieu G, Racine E. Preparing the ethical future of deep brain stimulation. Surg Neurol. Dec 2009; 72(6): 577–586; discussion 586. [DOI] [PubMed] [Google Scholar]
- 50. Rabins P, Appleby BS, Brandt J, DeLong MR, Dunn LB, Gabriels L., Greenberg BD, Haber SN, Holtzheimer PE 3rd, Mari Z., et al. Scientific and ethical issues related to deep brain stimulation for disorders of mood, behavior, and thought. Arch Gen Psychiatry. Sep 2009; 66(9): 931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tomycz ND, Cheng BC, Cantella D, Angle C, Oh MY, Whiting DM. Pursuing new targets and indications for deep brain stimulation: considerations for device‐related clinical research in the United States. Neuromodulation. Jul–Aug 2011; 14(4): 389–392. [DOI] [PubMed] [Google Scholar]
- 52. Fins JJ. Deep brain stimulation, free markets and the scientific commons: is it time to revisit the Bayh‐Dole act of 1980? Neuromodulation. Jul 2010; 13(3): 153–159. [DOI] [PubMed] [Google Scholar]
- 53. Racine E, Waldman S, Palmour N, Risse D, Illes J. “Currents of hope”: neurostimulation techniques in U.S. and U.K. print media. Camb Q Healthc Ethics. Summer 2007; 16(3): 312–316. [PubMed] [Google Scholar]
- 54. Valldeoriola F, Morsi O, Tolosa E, Rumia J, Marti MJ, Martinez‐Martin P. Prospective comparative study on cost‐effectiveness of subthalamic stimulation and best medical treatment in advanced Parkinson's disease. Mov Disord. Nov 15 2007; 22(15): 2183–2191. [DOI] [PubMed] [Google Scholar]
- 55. Meissner W, Schreiter D, Volkmann J, Trottenberg T, Schneider GH, Sturm V, Deuschl G, Kupsch A. Deep brain stimulation in late stage Parkinson's disease: a retrospective cost analysis in Germany. J Neurol. Feb 2005; 252(2): 218–223. [DOI] [PubMed] [Google Scholar]
- 56. Fraix V, Houeto JL, Lagrange C, Le Pen C, Krystkowiak P, Guehl D, Ardouin C, Welter ML, Maurel F, Defebvre L. et al. Clinical and economic results of bilateral subthalamic nucleus stimulation in Parkinson's disease. J Neurol Neurosurg Psychiatry. Apr 2006; 77(4): 443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]


