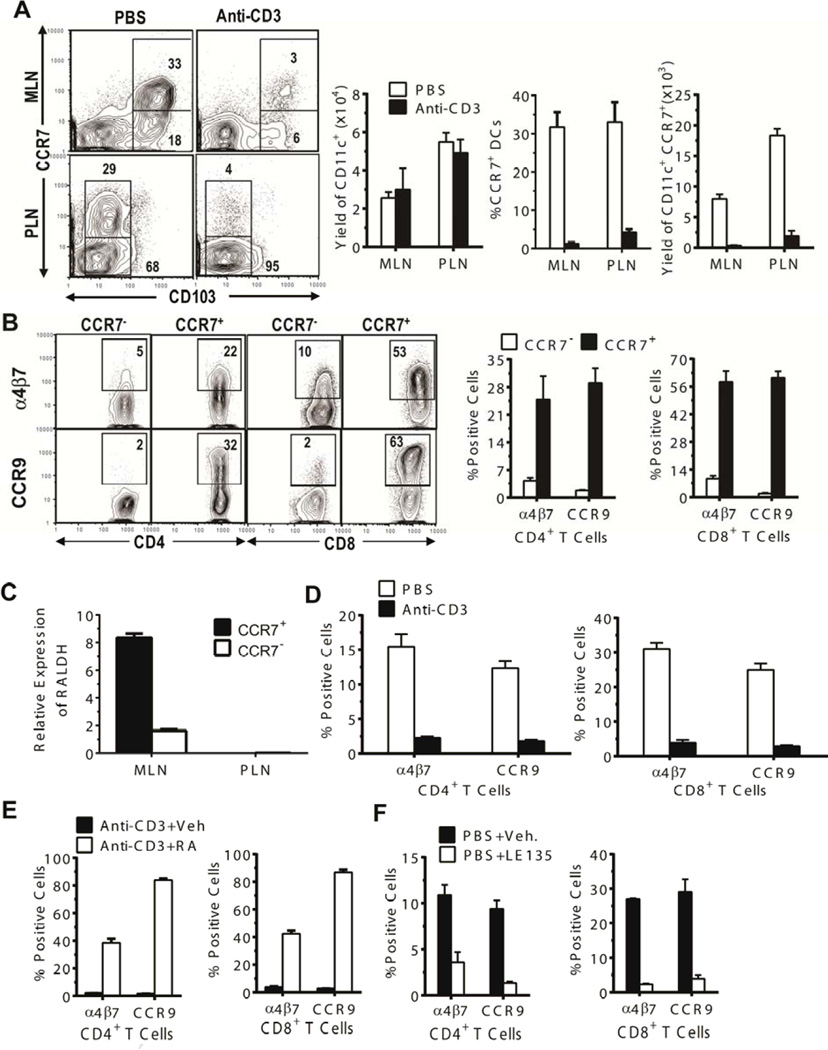
Figure 1. CCR7+ DC subset in MLN was necessary for induction of gut-homing receptor expression by donor T cells.
(A) Mononuclear cells of MLN and PLN from BALB/c mice 7 days after treatment with PBS or anti-CD3 were stained for CD11c, CCR7, and CD103. Gated CD11c+ cells are shown as CCR7 versus CD103 staining. A representative flow cytometry pattern and the percentage and yield (mean ± SE, N=4) of CCR7+ DC subsets and total CD11c+ cell yield from 1 of 4 replicate experiments are shown. (B) CD11c+CD103+ DCs from MLN of untreated BALB/c mice were sorted into CCR7+ and CCR7− subsets. Each subset (0.05 × 106) was co-cultured with C57BL/6 T cells (0.1 × 106) for 4 days. Thereafter, cultured cells were stained with CD4, CD8, α4β7, and CCR9. Gated CD4+ or CD8+ T cells are shown as CD4 or CD8 vs α4β7 or CCR9 staining. A representative flow cytometry pattern and the percentages (mean ± SE) of α4β7+ or CCR9+ cells among CD4+ or CD8+ T cells from 1 of 3 replicate experiments are shown. (C) Total RNA was isolated from sorted CCR7+ and CCR7− DCs from MLN and PLN of untreated BALB/c mice, and RALDH expression was analyzed by real-time PCR. Relative expression levels (mean ± SE) from 3 replicate experiments are shown. (D–F) Seven days after treatment with PBS or anti-CD3, CD11c+ DCs from host-type BALB/c MLN were sorted. The sorted CD11c+ cells (0.1 × 106) from PBS- or anti-CD3-treated mice were first co-cultured with splenic CD4+ and CD8+ T cells (0.2 × 106) from donor-type C57BL/6 (D); then, the sorted CD11c+ DCs from anti-CD3-treated mice were co-cultured with the donor T cells in the presence or absence of RA (E); finally, the sorted CD11c+ DCs from PBS-treated control mice were cultured with the donor T cells in the presence or absence of RA antagonist LE135 (1 µM) (E). After culture for 4 days, expression of α4β7 and CCR9 by the cultured T cells was analyzed by flow cytometry. Percentages (mean ± SE, N = 4) of α4β7+ or CCR9+ cells among CD4+ or CD8+ T cells are shown.