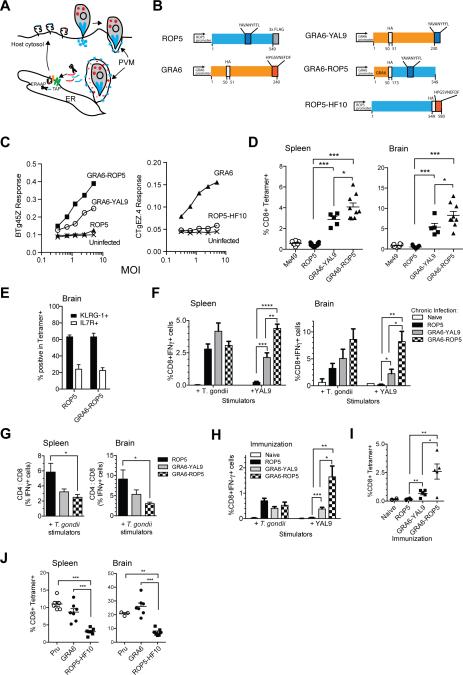
Figure 5. Enhanced T cell response when the antigenic precursor proteins are targeted to parasite dense granules.
A) Schematic depicting distinct modes of secretion for the dense granule protein GRA6 (depicted in red) (Holtappels et al., 2008), versus rhoptry protein ROP5 (depicted in blue). Also shown are possible routes for antigen processing and presentation via MHC-I. T. gondii injects rhoptry proteins directly into the host cell during invasion. ROP5 associates with the cytosolic face of parasitophorous vacuole membrane (PVM) following secretion. Parasites constitutively secrete dense granule proteins into the PV lumen of invaded host cells. Proteolytic processing of parasite secretory proteins could take place in the host cells' cytosol by the proteasome, and further trimming by ERAAP may occur after TAP-mediated transport of peptides into the ER. Processed peptides are loaded onto MHC class I molecules to be transported to the cell surface for recognition by a CD8 T cell (not depicted). B) Left hand diagrams show wild type ROP5 and GRA6 genes indicating the location of their antigenic epitopes (YAL9 and HF10) and placement of HA and FLAG epitope tags. Right hand diagrams show the transgenic constructs used to retarget expression of the epitopes to different parasite secretory compartments. GRA6-YAL9 has the HF10 epitope of the GRA6 gene replaced by YAL9. An additional 10 amino acids from ROP5 flanking the YAL9 epitope were included to allow for efficient processing (not depicted). GRA6-ROP5 contains the GRA6 promoter and signal sequence fused to the C-terminal portion of the ROP5IIC gene including the YAL9 epitope. ROP5-HF10 contains the HF10 epitope from GRA6 fused to the C-terminus of ROP5IIIC gene. All constructs were introduced as transgenes into the parental type III strain, CTG, which harbors allelic forms of ROP5 and GRA6 that lack the T cell stimulatory epitopes. C) BMDCs from C57BL/6 (H-2b) or B10.D2 (H-2d) mice were infected in vitro with irradiated transgenic T. gondii tachyzoites at varying MOI and BTg45Z and CTgEZ.4 lacZ response was measured after an overnight stimulation with BMDCs +/- T. gondii. D) C57BL/6 mice (H-2b) were infected i.p. with 105 transgenic T. gondii tachyzoites. Splenocytes or brain leukocytes were harvested from mice 3-4 wks post infection and T. gondii-specific T cell responses were measured by staining with tetramers. Compiled data showing MHC class I H-2Db–YAL9 tetramer staining on gated CD8+ B220-splenocytes (left panel) and brain leukocytes (right panel). F) Compiled data showing splenic (left panel) and brain (right panel) T. gondii-specific and H-2Db-YAL9-specific CD8 T cell responses from infected mice as measured by intracellular cytokine staining for IFN-γ. Data are corrected for background based on responses by T cells towards uninfected APCs or APCs pulsed with irrelevant peptide. G) Plots depicting the ratio of T. gondii-specific CD4 to CD8 T cell response from spleen (left panel) or brain (right panel) of infected mice. H) Mice were immunized with irradiated 5×106 transgenic T. gondii tachyzoites. Splenocytes were harvested from mice 2 wks post immunization and T. gondii-specific T cell responses were measured using intracellular IFN-γ staining for. Compiled data showing splenic T. gondii and YAL9-specific CD8 T cell responses from immunized mice. Data are corrected for background based on responses by T cells towards uninfected APCs or APCs pulsed with irrelevant peptide. (I) Compiled data showing MHC class I H-2Db–YAL9 tetramer staining on splenocytes from immunized mice. Cells were also co-stained with CD8 antibody. Each bar represents an average from six mice. Each dot represents an individual mouse. Data are pooled from two independent experiments. (J) B6×B6.C (H-2b/d) mice were infected with the indicated transgenic T. gondii tachyzoites. Splenocytes or brain leukocytes were harvested from mice 21 days post infection and GRA6-specific T cell responses were measured by staining with H-2Ld-HF10 tetramers. Compiled tetramer staining data on gated CD8+ B220- splenocytes (left panel) and CD8+ brain leukocytes (right panel). (*=p<0.05, **=p<0.01, ***=p<0.001,****=p<0.0001). See also Figure S4 and S5.