Fig. 5.
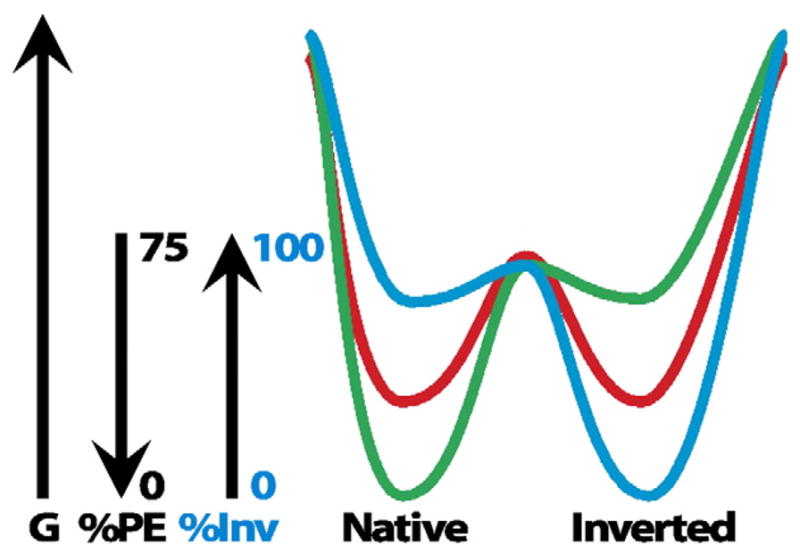
Dual minima energy folding funnel for LacY as a function of membrane lipid composition. The schematic depicts a two-dimensional section through the three-dimensional protein folding funnel. The folding of LacY to its lowest free energy state (G) proceeds via a funnel-shaped energy landscape whose shape is defined by the physicochemical properties of the lipid environment (green, 75% PE; red, intermediate % PE; blue, 0% PE). The topology of the N-terminal bundle of LacY is responsive to the membrane PE content. During initial folding of LacY multiple topological conformers are in rapid equilibrium, but subsequent folding events result in a stable mixture of conformers determined by the percent PE and separated by a high activation energy. A change in the PE content raises the free energy of the conformers resulting in a redistribution of conformers governed by the new PE content. This figure was originally published in [60] © the American Society for Biochemistry and Molecular Biology.
