Abstract
PHACE syndrome represents the association of large infantile hemangiomas of the head and neck with brain, cerebrovascular, cardiac, ocular, and ventral/midline defects. Cardiac and cerebrovascular anomalies are the most common extracutaneous features of PHACE, and they also constitute the greatest source of potential morbidity. Congenital heart disease in PHACE is incompletely described, and this study was conducted to better characterize its features. This study of the International PHACE Syndrome Registry represents the largest central review of clinical, radiology, and pathology data for cardiovascular anomalies in PHACE patients to date. 62/150 (41%) subjects had intracardiac, aortic arch, or brachiocephalic vessel anomalies. Aberrant origin of a subclavian artery was the most common cardiovascular anomaly (present in 31/150 (21%) of subjects). Coarctation was the second most common anomaly, identified in 28/150 (19%), and can be missed clinically in PHACE patients because of the frequent association of arch obstruction with aberrant subclavian origin. 23/62 (37%) subjects with cardiovascular anomalies required procedural intervention. A higher percentage of hemangiomas were located on the left side of the head/neck in patients with coarctation (46% vs. 39%); however, hemangioma distribution did not predict the presence of cardiovascular anomalies overall. In conclusion, PHACE is associated with a high risk of congenital heart disease. Cardiac and aortic arch imaging with detailed assessment of arch patency and brachiocephalic origins is essential for any patient suspected of having PHACE. Longitudinal investigation is needed to determine the long-term outcomes of cardiovascular anomalies in PHACE.
Keywords: PHACE syndrome, congenital heart disease, cardiovascular anomalies, coarctation of the aorta
The PHACE acronym was coined in 1996 to describe the association among malformations of the Posterior fossa, Hemangiomas of the head and neck, Arterial, Cardiovascular, and Eye anomalies, and ventral developmental defects).1-3 Cervical and cerebral arterial anomalies are the most common extracutaneous manifestations, occurring in 91%.4,5 Although cardiac anomalies are less common, they constitute the greatest source of potential morbidity and often require early surgical intervention. In addition, the co-occurrence of cardiac anomalies with cervical and cerebral arterial anomalies significantly increases the risk of acute ischemic stroke in PHACE.6-8 Various cardiovascular anomalies have been reported in PHACE.3,9-19 This study reviews the clinical, radiologic, and pathologic features of associated cardiac, aortic arch, and vascular bed anomalies in patients with known PHACE enrolled in a large international registry. Our aim is to better define the incidence, characterize the clinical and radiologic features of, and provide follow-up data for cardiovascular anomalies in PHACE.
Methods
A total of 155 patients with definite PHACE were identified from the IRB-approved PHACE Syndrome International Clinical Registry and Genetic Repository housed at the Medical College of Wisconsin/Children's Hospital of Wisconsin. All available clinical, radiology, and pathology data for anatomic abnormalities of the heart, aortic arch, brachiocephalic vessels, and cerebrovascular bed was reviewed by a team of pediatric dermatologists and a pediatric cardiologist from the Medical College of Wisconsin. Five subjects were excluded due to insufficient records (Figure 1). Patent foramen ovale (PFO) was considered a normal variant and excluded. A patent ductus arteriosus (PDA) was considered pathologic if it persisted past the neonatal period and was not associated with significant aortic arch obstruction or complex heart disease. Pearson chi-square and Fisher's exact tests were used to analyze data for hemangioma location and associated cardiovascular anomalies. Follow-up information (>2 years) was available for 10 subjects with coarctation cared for at the Medical College of Wisconsin, allowing for preliminary assessment of aortic disease progression over time.
Figure 1.
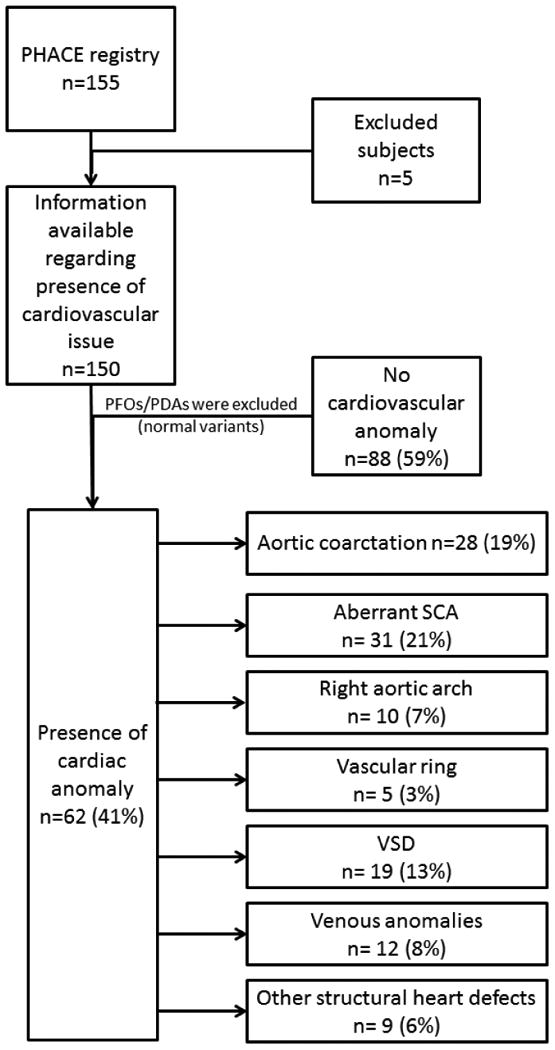
Flowchart demonstrating subject inclusion process and cardiovascular anomaly frequencies. Individual subjects may have more than 1 anomaly. Values are expressed as percent of total eligible registry population (n=150). PFO, patent foramen ovale; PDA, patent ductus arteriosus; SCA, subclavian artery; VSD, ventricular septal defect.
Aortic coarctation histologic specimens from surgical resection were available for 7 of the 17 PHACE patients who had aorta removed during aortic arch reconstruction. Paraffin blocks were available for 3/7 PHACE cases and 4/7 had only H&E slides for review. These samples were reviewed centrally at the Medical College of Wisconsin and compared to control specimens from 52 consecutive surgical coarctation repairs (non-PHACE) performed at Children's Hospital of Wisconsin.
Results
Figure 1 summarizes the cardiovascular anomalies: 62/150 (41%) PHACE patients had associated intracardiac, aortic arch, and/or brachiocephalic vessel anomalies. There were 51 (82%) females and 11 males (18%) with cardiovascular anomalies giving a female-to-male ratio of 4.6:1. This ratio was similar to that of the entire registry (4.2:1). Importantly, 57/62 (92%) with cardiovascular anomalies also had associated cervical or cerebral arterial anomalies (dysgenesis, narrowing, nonvisualization, primitive embryonic carotid-vertebrobasilar connections, and/or anomalous origin or course). In total, 23/62 subjects (37%) required procedural/surgical intervention for their cardiovascular anomalies.
Coarctation/interrupted aortic arch was found in 28/62 (45%), characterized by unusual long segment transverse arch narrowing with adjacent segments of aneurysmal dilatation that is quite distinctive from the typical isolated juxtaductal coarctation anatomy seen in individuals without PHACE (Figure 2). None of the PHACE patients' aortic arch defects were associated with bicuspid aortic valve, mitral valve anomalies, or left ventricular hypoplasia. Intervention for significant aortic arch obstruction was required in 17/28 (61%) subjects with coarctation of the aorta, and the age range for the intervention was 4 days-3.5 years with a median age of 2 months. Three individuals required interposition grafts as infants because of the long-segment nature of the obstruction.
Figure 2.
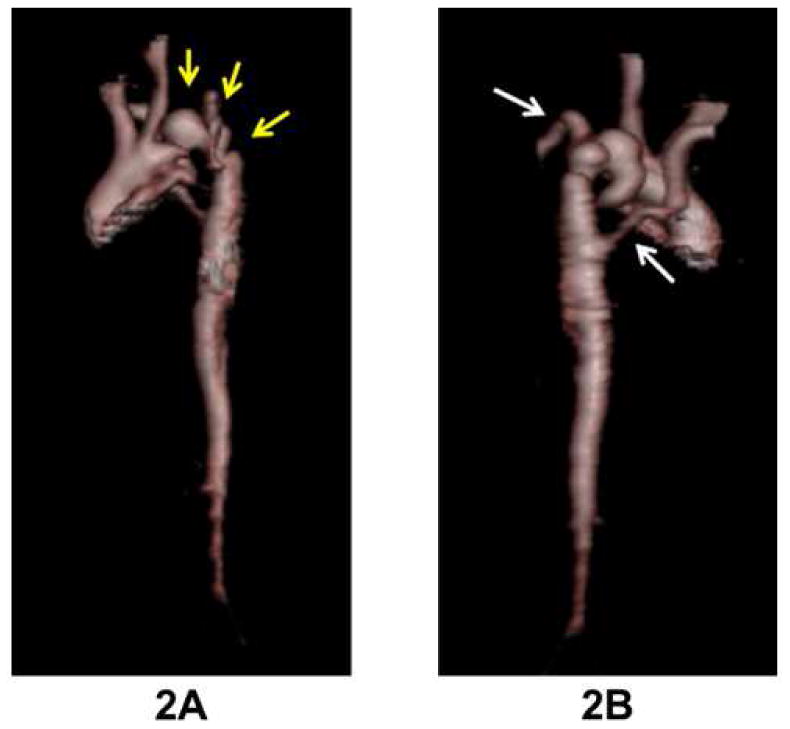
Three dimensional MRI aortic reconstruction in this PHACE patient with severe coarctation with multiple areas of narrowing and aneurysms in the transverse arch (as outlined by arrows in 2A). Both subclavian arteries arise distal to areas of obstruction (arrows in 2B), and the right subclavian artery has an aberrant origin.
Of the 10 individuals with coarctation and follow-up data available, 7 have undergone surgical repair and 3 have been followed without intervention because of a mild gradient at presentation. Mean age at follow-up was 8 years (range 3-15 years). All of the patients without intervention have continued to demonstrate only mild arch gradients without progression, and none have required late intervention. Of the subjects who had surgery, 5/7 have stable aortic arch patency without progressive narrowing/dilation and have not required re-intervention. The 2 children who required interposition graft placement as infants have developed expected progressive narrowing with growth, and 1 graft has been replaced at 11 years of age.
Brachiocephalic abnormalities were the most common cardiovascular anomaly, identified in 35/62 (56%) subjects. Aberrant origin of a subclavian artery (SCA) was found in 31/62 (50%), with 22 having aberrant origin of the right SCA from a left aortic arch, 8 having aberrant origin of a left SCA from a right aortic arch, and 1 having an aberrant left SCA origin from a left aortic arch. Of the 28 subjects with coarctation, 16 (57%) also had aberrant SCA origin so that both SCAs arose distal to the obstruction. Origin of both carotid arteries from a common trunk (“bovine” arch) was identified in 5/62 (8%), and 1 subject had isolated aneurysmal dilatation of the right SCA. Abnormal aortic arch sidedness was seen in 10/62 (16%) subjects having a dominant right aortic arch. A vascular ring was present in 5 subjects (all with a dominant right aortic arch), and 3 required surgical division. Finally, 7 subjects had ascending aortic dilatation without evidence of aortic valve pathology.
Intracardiac anomalies were also found in the cohort, with 19/62 (31%) having a ventricular septal defect (VSD). Isolated VSDs without associated intracardiac abnormalities were most common (16/19), and they frequently did not require surgical intervention (8/8 muscular VSDs and 5/8 perimembranous VSDs closed spontaneously). Complex congenital heart disease was rarely seen (3/62), with 2 cases of tetralogy of Fallot and 1 case of tricuspid atresia, as were other intracardiac heart defects (2 pulmonary stenosis, 1 atrial septal defect, 1 PDA, and 1 bicuspid aortic valve without arch anomalies). 1 subject had dextrocardia with a left-sided aortic arch and situs solitus without other cardiovascular anomalies. In addition, superior systemic venous anomalies were identified in 12/62 (19%), with 7 having a retroaortic innominate vein and 5 having bilateral superior vena cavae with a left superior vena cava draining to the coronary sinus.
Histologic examination of surgically excised aortic segments demonstrated strikingly similar abnormalities in the 7 PHACE specimens. In 5/7 specimens, there were large, well-delineated mural zones of scarring and necrosis with almost complete loss of arterial smooth muscle cells and elastic fibers in the intima and media (Figure 3). The remaining 2 PHACE coarctation segments had smaller areas of decreased arterial smooth muscle cells in the tunica media and evidence of increased adventitial collagen deposition.
Figure 3.
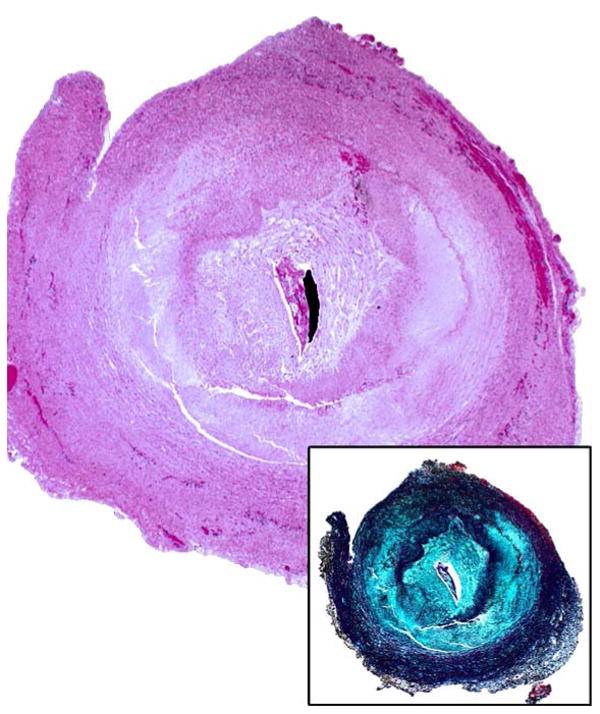
Low power H&E of aortic coarctation tissue from a PHACE patient demonstrating intimal hyperplasia and large arcuate zones with loss of smooth muscle and elastic tissue. Inset shows elastin stain.
The excised aortic segments from patients without PHACE were notably different from those with PHACE, with 47/52 (90%) of the non-PHACE coarctation control specimens exhibiting typical coarctation findings with a short, asymmetrical post-ductal narrowing, often viewed as an intimal shelf. In addition, there was intimal fibrosis and tunica media showing multiple fascicles of muscular elastic tissue occasionally extending from the ductus onto the adjacent aortic wall. Only 5/52 non-PHACE segments contained zones of medial necrosis similar to the PHACE specimens, and 3 of those were likely related to suture scar formation from previous surgical intervention. Although the adventitial layer appeared thicker in the PHACE specimens compared to the non-PHACE specimens, the majority of excised segments in both cohorts lacked extra-adventitial soft tissues so that analysis of adventitial thickness was difficult.
All PHACE patients with cardiovascular anomalies had a segmental infantile hemangioma of the head and neck region. A higher percentage of infantile hemangiomas were located on the left side of the head/neck in patients with coarctation (46%) when compared to those without coarctation in our registry population (39%) (p=0.048). However, hemangioma laterality (left, right, or bilateral) did not predict the presence of congenital heart disease overall (p=0.243). Additionally, there was no ipsilateral association between the aortic arch direction and cervicofacial hemangioma location. Of the 10 patients with a right-sided aortic arch, 4 had right-sided IH, 2 IH were left-sided, and 2 were bilateral (p=0.216). Hemangioma distribution involving the mandibular (S3) facial segment20 was not associated with cardiovascular anomalies, as 47% of subjects with S3 involvement had cardiovascular anomalies versus 38% of those without S3 involvement (p=0.316). The presence of a midline anomaly (sternal cleft or median raphe) did not significantly differ between subjects with (31%) or without (24%) congenital heart disease (p=0.448). Additionally, there did not seem to be differences in the prevalence of other features of PHACE.
Discussion
This study is the largest comprehensive investigation to date addressing cardiovascular anomalies in PHACE. Cardiovascular anomalies were present in 41% of our PHACE cohort. Previous publications have reported an incidence of cardiovascular anomalies in PHACE ranging from 21-67%.2,4,21-23 Prior studies have used variable definitions of cardiovascular anomalies, often including normal cardiac variants such as PFO or PDA. In addition, these studies lacked detailed assessment of the brachiocephalic arterial and venous vessels and had fewer subjects than our cohort. The incidence of cardiovascular anomalies in PHACE is significantly higher than the general population and many other syndromes well-known to be associated with congenital heart disease. The 19% incidence of coarctation in our cohort is higher than reported in Turner syndrome (6.9-15.7%)24,25 and syndromes associated with microdeletions of chromosome 22q11 (DiGeorge syndrome, velocardiofacial syndrome, and conotruncal anomaly face syndrome). Brachiocephalic vessel and aortic arch sidedness abnormalities are also much more common in PHACE than the general population. A review of CT angiograms of 1005 adults found aberrant subclavian artery in 1.2%, right aortic arch in 0.1%, and systemic venous anomalies in 0.7% (compared to 21%, 7%, and 8% in our cohort, respectively).26
Coarctation or interrupted aortic arch in PHACE is unique and complex, both in its location and character, compared to typical coarctation anatomy. The coarctation is most often in the transverse aortic arch, rather than the aortic isthmus, and can be characterized by multiple areas of long segment arch narrowing with adjacent segments of bizarre aneurysmal dilatation (Figure 2). Also notable is the absence of associated left heart valve pathology classically seen with coarctation, emphasized by the fact that none of our PHACE subjects with coarctation had aortic valve or mitral valve anomalies (compared to the 50-80% incidence of bicuspid aortic valve associated with typical coarctation).11 Most importantly, these unusual and severe aortic arch anomalies may be difficult to appreciate clinically in PHACE patients due to the commonly associated aberrant SCA origin, as almost 60% of our cohort has both subclavian arteries arise distal to the obstruction (making 4 extremity blood pressure assessment of obstruction ineffective in identifying the arch gradient). Follow-up data for a small subset of PHACE patients with coarctation cared for at the Medical College of Wisconsin did not show evidence of aortic arch narrowing; however, longer-term data from larger numbers of patients is needed to determine risk for progression. The PHACE Syndrome International Clinical Registry will facilitate longitudinal evaluation of PHACE patients with coarctation and other cardiovascular anomalies into adolescence and adulthood.
Histopathologic examination of affected aortic tissue from 7 of our PHACE subjects demonstrated markedly similar zonal bands of smooth muscle loss, elastin loss, and fibrosis of the inner tunica media. These findings parallel other histologic descriptions of affected aortic and arterial tissue in PHACE.9,10,13,19 With extensive post-mortem microscopic examination in a PHACE patient with coarctation, Chad et al. identified widespread aortic abnormalities including medial changes and fibro-intimal thickening.13 Additionally, their case report identified diffuse arteriopathy in medium to large arteries without venous or small vessel disease found on histopathology. Although our series identified a few patients with structural venous anomalies, the clinical, radiologic, and histopathologic evidence suggests that the primary vascular defect in PHACE is developmental dysplasia of large and medium sized arteries. The identification of ascending aortic dilatation without aortic valve disease also suggests a primary vasculopathy and emphasizes the need for long-term major artery surveillance of all PHACE patients.
Previous studies have suggested that IH location could predict the risk for associated anomalies.4,23,27 A hemangioma pattern map with four facial segments corresponding to developmental facial prominences has been developed.20 Prior publications have noted an ipsilateral association between aortic arch direction and cervicofacial hemangioma laterality9,10 and hypothesized a relationship between mandibular hemangioma distribution and cardiovascular or midline anomalies.23,27 Neither pattern was supported by our data. Although a higher percentage of hemangiomas were located on the left side of the head/neck in patients with coarctation (46% vs. 39%), hemangioma distribution did not predict the presence of cardiovascular anomalies overall. Hemangioma location should not dictate screening exams; all children with large segmental hemangiomas of the head and neck need a careful cardiac and aortic arch assessment. A comprehensive evaluation for a patient at risk for PHACE syndrome includes a history and physical with special attention for midline defects (sternal pits, tags, and clefts or supraumbilical raphe), an ocular exam, structural brain imaging, cervical/cerebral arterial and aortic arch/brachiocephalic imaging with magnetic resonance angiography, and intracardiac imaging using echocardiography.
Limitations in this study included the retrospective study design and lack of longitudinal data on some patients. Some of the subjects enrolled in our registry have been previously reported in the literature. Although it is possible that patients with more complex anomalies may be referred to our tertiary institution or to the registry, reviewing the entire registry population provided a large sample size and allowed us to approximate the incidence of cardiovascular anomalies in PHACE.
Acknowledgments
We acknowledge Shawna Joachim for her work with the PHACE Syndrome International Clinical Registry and Genetic Repository.
Support/Grant Information: Grant support for the PHACE Syndrome International Clinical Registry and Genetic Repository was provided by the Greater Milwaukee Foundation (Milwaukee, WI, USA).
Statistical analysis was provided by the Clinical and Translational Science Institute of Southeast Wisconsin (Milwaukee, WI, USA). Statistical analysis for this project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number 8UL1TR000055. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
J.T. Shieh receives support from the National Institutes of Health, National Heart Lung, and Blood Institute, Grant HL092970.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pascual-Castroviejo I. Vascular and nonvascular intracranial malformation associated with external capillary hemangiomas. Neuroradiology. 1978;16:82–84. doi: 10.1007/BF00395211. [DOI] [PubMed] [Google Scholar]
- 2.Frieden IJ, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol. 1996;132:307–311. doi: 10.1001/archderm.132.3.307. [DOI] [PubMed] [Google Scholar]
- 3.Schneeweiss A, Blieden LC, Shem-Tov A, Motro M, Feigel A, Neufeld HN. Coarctation of the aorta with congenital hemangioma of the face and neck and aneurysm or dilatation of a subclavian or innominate artery. A new syndrome? Chest. 1982;82:186–187. doi: 10.1378/chest.82.2.186. [DOI] [PubMed] [Google Scholar]
- 4.Haggstrom AN, Garzon MC, Baselga E, Chamlin SL, Frieden IJ, Holland K, Maguiness S, Mancini AJ, McCuaig C, Metry DW, Morel K, Powell J, Perkins SM, Siegel D, Drolet BA. Risk for PHACE syndrome in infants with large facial hemangiomas. Pediatrics. 2010;126:e418–426. doi: 10.1542/peds.2009-3166. [DOI] [PubMed] [Google Scholar]
- 5.Hess CP, Fullerton HJ, Metry DW, Drolet BA, Siegel DH, Auguste KI, Gupta N, Haggstrom AN, Dowd CF, Frieden IJ, Barkovich AJ. Cervical and intracranial arterial anomalies in 70 patients with PHACE syndrome. AJNR Am J Neuroradiol. 2010;31:1980–1986. doi: 10.3174/ajnr.A2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel DH, Tefft KA, Kelly T, Johnson C, Metry D, Burrows P, Pope E, Cordisco M, Holland KE, Maheshwari M, Keith P, Garzon M, Hess C, Frieden IJ, Fullerton HJ, Drolet BA. Stroke in children with posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities (PHACE) syndrome: a systematic review of the literature. Stroke. 2012;43:1672–1674. doi: 10.1161/STROKEAHA.112.650952. [DOI] [PubMed] [Google Scholar]
- 7.Drolet BA, Dohil M, Golomb MR, Wells R, Murowski L, Tamburro J, Sty J, Friedlander SF. Early stroke and cerebral vasculopathy in children with facial hemangiomas and PHACE association. Pediatrics. 2006;117:959–964. doi: 10.1542/peds.2005-1683. [DOI] [PubMed] [Google Scholar]
- 8.Burrows PE, Robertson RL, Mulliken JB, Beardsley DS, Chaloupka JC, Ezekowitz RA, Scott RM. Cerebral vasculopathy and neurologic sequelae in infants with cervicofacial hemangioma: report of eight patients. Radiology. 1998;207:601–607. doi: 10.1148/radiology.207.3.9609880. [DOI] [PubMed] [Google Scholar]
- 9.Bronzetti G, Giardini A, Patrizi A, Prandstraller D, Donti A, Formigari R, Bonvicini M, Picchio FM. Ipsilateral hemangioma and aortic arch anomalies in posterior fossa malformations, hemangiomas, arterial anomalies, coarctation of the aorta, and cardiac defects and eye abnormalities (PHACE) anomaly: report and review. Pediatrics. 2004;113:412–415. doi: 10.1542/peds.113.2.412. [DOI] [PubMed] [Google Scholar]
- 10.Prada F, Mortera C, Bartrons J, Rissech M, Jimenez L, Carretero J, Rovira C, Vicente MA. Complex aortic coarctation and PHACE syndrome. Rev Esp Cardiol. 2010;63:1367–1370. doi: 10.1016/s1885-5857(10)70261-9. [DOI] [PubMed] [Google Scholar]
- 11.Rao RP, Drolet BA, Holland KE, Frommelt PC. PHACES association: a vasculocutaneous syndrome. Pediatr Cardiol. 2008;29:793–799. doi: 10.1007/s00246-008-9204-5. [DOI] [PubMed] [Google Scholar]
- 12.Bijulal S, Sivasankaran S, Krishnamoorthy KM, Titus T, Tharakan JA, Krishnamanohar SR. Unusual coarctation-the PHACE syndrome: report of three cases. Congenit Heart Dis. 2008;3:205–208. doi: 10.1111/j.1747-0803.2008.00193.x. [DOI] [PubMed] [Google Scholar]
- 13.Chad L, Dubinski W, Hawkins C, Pope E, Bernstein S, Chiasson D. Postmortem vascular pathology in PHACES syndrome: a case report. Pediatr Dev Pathol. 2012;15:507–510. doi: 10.2350/12-05-1203-CR.1. [DOI] [PubMed] [Google Scholar]
- 14.Giardini A, Gholam C, Khambadkone S, Kostolny M. Need for comprehensive vascular assessment before surgical repair of aortic coarctation in PHACES syndrome. Pediatr Cardiol. 2010;31:291–293. doi: 10.1007/s00246-009-9592-1. [DOI] [PubMed] [Google Scholar]
- 15.Wendelin G, Kitzmuller E, Salzer-Muhar U. PHACES: a neurocutaneous syndrome with anomalies of the aorta and supraaortic vessels. Cardiol Young. 2004;14:206–209. doi: 10.1017/S1047951104002173. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Gutierrez JC. PHACES syndrome and ectopia cordis. Interact Cardiovasc Thoracic Surg. 2011;12:642–644. doi: 10.1510/icvts.2010.258442. [DOI] [PubMed] [Google Scholar]
- 17.Yates R, Syed S, Tsang V, Harper JI. Haemangioma of the head and neck with subglottic involvement and atypical coarctation. Br J Dermatol. 2000;143:686–688. doi: 10.1111/j.1365-2133.2000.03817.x. [DOI] [PubMed] [Google Scholar]
- 18.Wong CH, Wright JG, Silove ED, Willetts R, Brawn WJ. A new syndrome of multiple hemangiomas, right dominant double aortic arch, and coarctation. J Thorac Cardiovasc Surg. 2001;121:1207–1209. doi: 10.1067/mtc.2001.112627. [DOI] [PubMed] [Google Scholar]
- 19.Gargiulo G, Pace Napoleone C, Giardini A, Formigari R, Pierangeli A. Repair of a complex aortic arch anomaly associated with cutaneous hemangioma. Ann Thorac Surg. 2002;74:245–246. doi: 10.1016/s0003-4975(02)03554-3. [DOI] [PubMed] [Google Scholar]
- 20.Haggstrom AN, Lammer EJ, Schneider RA, Marcucio R, Frieden IJ. Patterns of infantile hemangiomas: new clues to hemangioma pathogenesis and embryonic facial development. Pediatrics. 2006;117:698–703. doi: 10.1542/peds.2005-1092. [DOI] [PubMed] [Google Scholar]
- 21.Metry D, Heyer G, Hess C, Garzon M, Haggstrom A, Frommelt P, Adams D, Siegel D, Hall K, Powell J, Frieden I, Drolet B, Conference PSR. Consensus Statement on Diagnostic Criteria for PHACE Syndrome. Pediatrics. 2009;124:1447–1456. doi: 10.1542/peds.2009-0082. [DOI] [PubMed] [Google Scholar]
- 22.Metry DW, Dowd CF, Barkovich AJ, Frieden IJ. The many faces of PHACE syndrome. J Pediatr. 2001;139:117–123. doi: 10.1067/mpd.2001.114880. [DOI] [PubMed] [Google Scholar]
- 23.Metry DW, Haggstrom AN, Drolet BA, Baselga E, Chamlin S, Garzon M, Horii K, Lucky A, Mancini AJ, Newell B, Nopper A, Heyer G, Frieden IJ. A prospective study of PHACE syndrome in infantile hemangiomas: demographic features, clinical findings, and complications. Am J Med Genet A. 2006;140:975–986. doi: 10.1002/ajmg.a.31189. [DOI] [PubMed] [Google Scholar]
- 24.Kim HK, Gottliebson W, Hor K, Backeljauw P, Gutmark-Little I, Salisbury SR, Racadio JM, Helton-Skally K, Fleck R. Cardiovascular anomalies in Turner syndrome: spectrum, prevalence, and cardiac MRI findings in a pediatric and young adult population. AJR Am J Roentgenol. 2011;196:454–460. doi: 10.2214/AJR.10.4973. [DOI] [PubMed] [Google Scholar]
- 25.Mazzanti L, Cacciari E. Congenital heart disease in patients with Turner's syndrome. Italian Study Group for Turner Syndrome (ISGTS) J Pediatr. 1998;133:688–692. doi: 10.1016/s0022-3476(98)70119-2. [DOI] [PubMed] [Google Scholar]
- 26.Berko NS, Jain VR, Godelman A, Stein EG, Ghosh S, Haramati LB. Variants and anomalies of thoracic vasculature on computed tomographic angiography in adults. J Comput Assist Tomogr. 2009;33:523–528. doi: 10.1097/RCT.0b013e3181888343. [DOI] [PubMed] [Google Scholar]
- 27.Oza VS, Wang E, Berenstein A, Waner M, Lefton D, Wells J, Blei F. PHACES association: a neuroradiologic review of 17 patients. AJNR Am J Neuroradiol. 2008;29:807–813. doi: 10.3174/ajnr.A0937. [DOI] [PMC free article] [PubMed] [Google Scholar]


