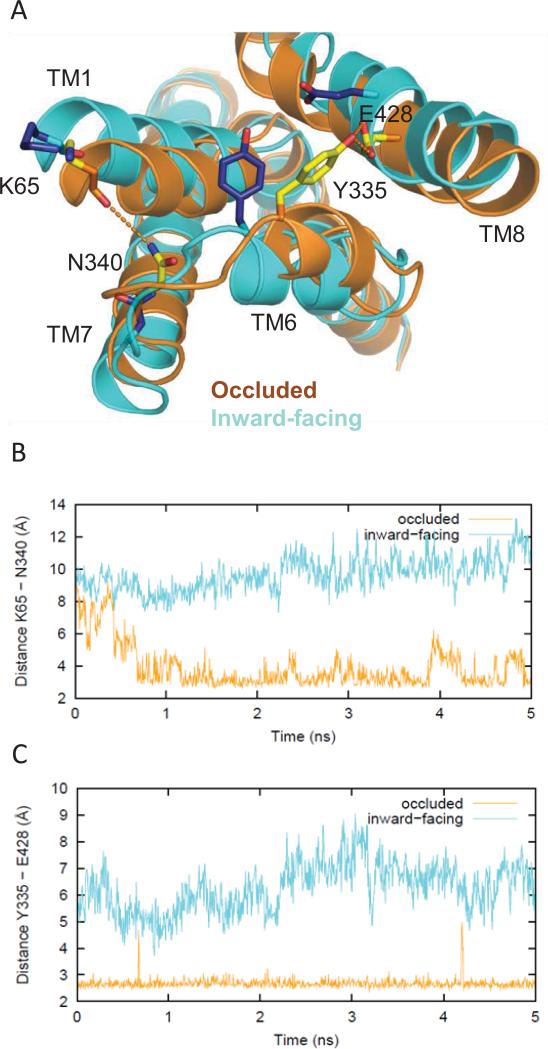
Figure 8.
Differences in the network of interactions in the occluded vs inward-facing states. (A) Tyr335 and Glu428 form an H-bond that helps stabilize the occluded conformation (yellow side chains, orange ribbons), but is not seen in the inward-facing conformation (blue side chains, cyan ribbons). Therefore, the Y335C mutant favors an inward-facing conformation over the occluded one, thus disrupting dopamine uptake. Similarly, Lys65 in TM1 and Asn340 in TM7 form an H-bond in the occluded conformation but do not interact in the inward-facing conformation (same color scheme as above). (B) Evolution of the changes in the H-bonding monitored by the minimum distances between the hydroxyl oxygen of Tyr335 and the carboxyl oxygens of Glu428 in the occluded (orange) and inward-facing conformations (cyan) in the last 5 ns of the equilibrated phase of the simulation. (C) The distances between the carbonyl oxygen of Lys65 and the side chain amide nitrogen of Asn340 in the occluded (orange) and inward-facing conformations (cyan) in the last 5 ns of the equilibrated phase of the simulation.