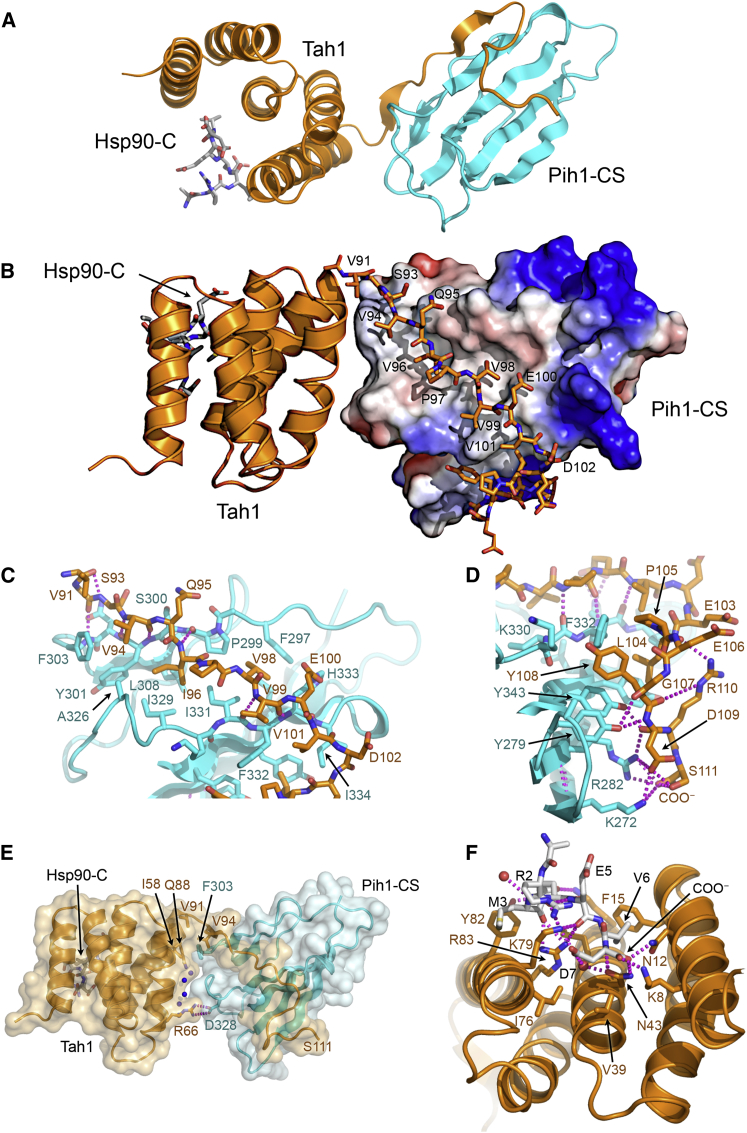
Figure 2.
Structure of Tah1- Pih1 Complex
(A) Secondary structure cartoon of TPR domain of Tah1 (gold) bound to the CS domain of Pih1 (cyan) and a peptide derived from the C terminus of Hsp90 (sticks).
(B) Overview of the Tah1-Pih1 complex showing the C-terminal segment of Tah1 (gold, carton and sticks) extending across a shallow hydrophobic depression on the edge of Pih1-CS domain (colored by electrostatic potential: +ve blue → −ve red), and wrapping round to contact the face of the four-stranded β sheet.
(C) The proximal part of the Tah1 C terminus segment bridging the edges of the two β sheets of the Pih1-CS domain β sandwich, with a combination of main chain to main chain hydrogen bonds and hydrophobic interactions.
(D) Interaction of the distal part of the Tah1 C terminus segment, forming a mixed hydrophobic and polar interface with the face of the four-stranded β sheet.
(E) Outside the substantial interaction mediated by the Tah1 C-terminal tail, the juxtaposition of the Tah1-TPR and Pih1-CS domains is fixed by an additional hydrophobic interaction, and an extended polar interface anchored by a bidentate hydrogen bond/ion-pair interaction.
(F) Interactions of the Tah1-TPR domain and Hsp90 C-terminal peptide. The Hsp90 peptide binds with compacted conformation stabilized by interaction of the peptide backbone and side chain of Glu 4, with the side chains of Tah1 residues Lys 50, Lys 79, and Arg 83. The α-carboxyl and carboxylate side chain of Asp 7 is bound by a “carboxylate clamp” formed by Tah1 residues Lys 8, Asn 12, Asn 43, and Lys 79. The side chain of Met 3 in the Hsp90 peptide packs against Tyr 82, but is far more exposed than those on other Hsp90-TPR-domain complexes.