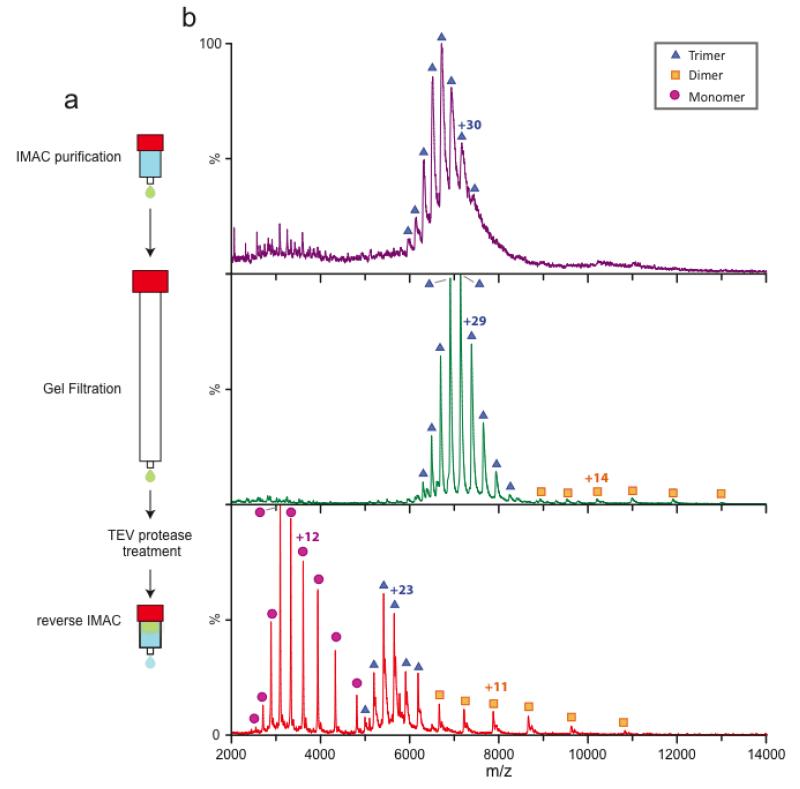
Figure 5. Additional purification steps lead to an improvement in the overall quality of mass spectra for AmtB.
The trimeric AmtB was expressed as a tobacco etch mosaic virus (TEV) protease cleavable N-terminal fusion to GFP and 6× His-tag (AmtB-GFP). (a) General outline for the purification of membrane protein complexes for native MS. Solubilized AmtB-GFP is first purified by IMAC then concentrated and further purified by gel filtration chromatography. The C-terminal fusion is removed by TEV protease treatment and further purified by reverse IMAC resulting in highly purified membrane protein complexes. (b) Native mass spectra at various time points in the purification process. After IMAC purification (top panel, purple) the ion peaks are broad for the trimeric complex with no disassociation products observed. Post gel filtration (middle panel, green) the ion peaks are improved for the trimeric complex along with disassociation products, monomer and dimer. The final purification step is removal of the C-terminal fusion which results in resolved mass spectra for both the trimeric complex and disassociation complexes (bottom panel, red). In general, we find that removal of the C-terminal fusion leads to more disassociation products.