Figure 1.
Known and Putative R2TP and R2TP-like Complexes
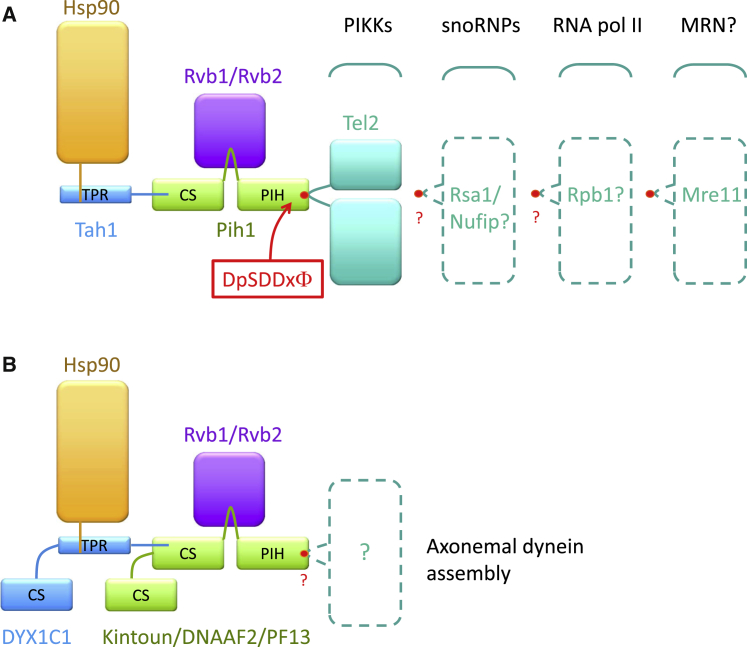
(A) The R2TP complex recruits PIKKs, snoRNPs, and RNA polymerase II to Hsp90. An adaptor protein, Tel2, is required for PIKK recruitment to Pih1 via a phosphoserine motif. Rsa1/Nufip has been proposed to play a similar role for snoRNPs; however, it is unknown whether RNA polymerase II requires a similar adaptor or whether subunits of the complex itself, such Rbp1, interact directly with Pih1. It is also unknown whether the same, or a similar, phosphoserine recognition motif is involved in these interactions (red circle). However, the phosphoserine motif is found in Mre11, which can associate with Pih1; thus, the MRN complex is a putative Hsp90-R2TP client.
(B) The complex between DYX1C1 and Kintoun/DNAAF2/PF13 may form an R2TP-like complex linking assembly of axonemal dynein to Hsp90. The protein that associates directly with R2TP to facilitate this interaction is unknown, and it is unknown whether a phosphoserine motif will mediate this.