Abstract
Purpose
Transformation of follicular lymphoma (FL) is a critical event associated with a poor prognosis. The role of the tumor microenvironment in previous transformation studies has yielded conflicting results.
Experimental Design
To define cell subtypes associated with transformation, we examined tissue specimens at diagnosis from patients with FL that later transformed and, using immunohistochemistry (IHC), stained for CD68, CD11c, CD21, CXCL13, FOXP3, PD-1 and CD14. Cell content and pattern of expression were evaluated. Those identified as significantly associated with time to transformation (TTT) and overall survival (OS) were further characterized by flow cytometry and multicolor IHC.
Results
58 patients were analyzed with median TTT of 4.7 years. The pattern of PD1+ and CD14+ cells rather than the quantity of cells was predictive of clinical outcomes. On multivariate analysis including the FLIPI score, CD14+ cells localized in the follicle were associated with a shorter TTT (HR=3.0; p=0.004). PD-1+ cells with diffuse staining were associated with a shorter TTT (HR=1.9; p=0.045) and inferior OS (HR=2.5; p=0.012). Multicolor IHC and flow cytometry identified CD14+ cells as follicular dendritic cells (FDC) while PD1+ cells represented two separate populations, TFH and exhausted T-cells.
Conclusion
These results identify the presence of PD1+ T-cells and CD14+ FDC as independent predictors of transformation in follicular lymphoma.
Keywords: CD14, PD1, follicular lymphoma, tumor microenvironment, time to transformation
Background
Follicular lymphoma is the second most common type of Non-Hodgkin lymphoma. With a median overall survival of nearly 10 years, follicular lymphoma is classically thought of an indolent lymphoma that exhibits periods of disease remission and stability punctuated by intermittent relapses (1). However, the disease course is often heterogeneous with some patients undergoing histologic transformation to an aggressive lymphoma, most often diffuse large B-cell lymphoma (DLBCL). Histologic transformation is often associated with rapid progression, refractoriness to treatment and an overall dismal prognosis (1–4). The incidence of transformation is variable ranging from 10–60% in different studies. The difference in incidence is largely due to differences in follow up, biopsy confirmation and inconsistent definitions of transformation (1, 3–8). The largest cohort reported an annual incidence of 3% (1). Prognostic tools utilizing clinical and laboratory factors have been developed such as the follicular lymphoma international prognostic index (FLIPI) score that can predict risk of transformation at diagnosis(3, 9).
Recent studies have demonstrated the prominent role the tumor microenvironment plays in disease severity and outcomes in follicular lymphoma(10). Gene expression profiling from the Leukemia/Lymphoma Molecular Profiling Project (LLMPP) identified the non-malignant microenvironment immune cells rather than the tumor cells as predictive of clinical outcomes and behavior. One expression signature, immune-response 1, seemed to be derived from reactive T-cells and was associated with a favorable outcome. The other expression profile, immune response-2, included genes preferentially expressed by macrophages and dendritic cells that were associated with inferior survival (11). IHC studies of the microenvironment have identified multiple immune subsets of interest (FOXP3+, PD1+, and CD4+/CD8+ ratio) that correlate with divergent outcomes(12–16). However, these studies have often analyzed a few different IHC markers at a time and many of the studies have had led to contradictory results (12, 17).
The association of an unfavorable outcome with genes expressed by macrophages and dendritic cells has led to increased interest in these immune subsets in follicular lymphoma patients. Farinha and others previously described that CD68+ macrophages or lymphoma associated macrophages (LAM) were correlated with inferior survival in their cohort (18), though this effect was demonstrated to be overcome with treatment with rituximab (19). CD14+ monocytes that are also HLA-DRlow have been shown to have immunosuppressive effects in various clinical conditions and several solids tumors (20–23). Lin and colleagues (24) recently described the role of CD14+ monocytes in patients with B-cell NHL. They showed that increased levels of CD14+ HLA-DRlow monocytes in the peripheral blood were associated with more advanced and aggressive disease and a shorter time to progression. Though these studies suggest an association of CD14+ cells with inferior outcomes they were based on peripheral blood and not tumor tissue. In addition, various other factors in the microenvironment such as PD1 expression have been identified as potentially impacting clinical outcomes in follicular lymphoma(13, 25). Recent studies have also demonstrated that the location of microenvironment cells with respect to the neoplastic follicle rather than the total cell quantity is predictive of clinical outcomes in follicular lymphoma (17).
We hypothesized that intratumoral cells expressing CD14 or PD1 would be associated with a shorter time to transformation in patients with follicular lymphoma. To this end, we studied the clinical correlation between the prevalence and distribution of various components of the tumor microenvironment, including CD14+ cells, CD68+ macrophages, FOXP3+ and PD1+ cells, and the time to transformation and overall survival in a retrospective cohort of transformed follicular lymphoma patients. Beyond identifying these cells of interest, we also attempted to better characterize and identify the underlying immune cell type through multicolor IHC and flow cytometry.
Methods
Patients
Patients with follicular lymphoma that later transformed to DLBCL were identified from the Mayo Clinic Lymphoma Database. All samples were from the time of diagnosis and confirmed by a hematopathologist (AF) to be follicular lymphoma. Transformation was confirmed by biopsy and all histologies at transformation were consistent with DLBCL. 58 patients with tissue available at diagnosis were identified and included in this analysis. Clinical characteristics including age, sex, presence of B symptoms, stage, grade and laboratory parameters were collected at time of diagnosis. The various components of the FLIPI score (age >60, Stage 3 or 4, Hgb <12 mg/dL, > 4 nodal areas involved and LDH > upper limit normal) were collected to calculate FLIPI score for each patient. This study was approved through the Mayo Clinic institutional review board.
Immunohistochemistry
Paraffin-embedded tissue was obtained from Mayo Clinic Tissue Registry and serial 5 µm sections were used for IHC. The tissue was de-paraffinized with three changes of xylene and cleared through graded series of ethanol. Endogenous peroxidase was quenched by incubation in 50% methanol/H2O2 and after rinsing with tap water, all sections were pretreated for 30 minutes with 50 mmol/L EDTA using a steamer and cooled for additional 5 minutes. All staining was done automatically on DAKO (Carpinteria, CA) Autostainer using the following antibodies to CD11c (Leica Microsystems 5D11), CD14 (Cell Marque EPR 3653), CD21(DAKO 1F8), CD68 (DAKO PG-M1), CXCL13 (R&D Systems 53610), FOXP3 (Abcam 236AE/7), and PD1 (Abcam NAT). The sections were viewed with an Olympus BXFA51 microscope and pictures taken with an Olympus DP71 camera. Slides were characterized by pattern of expression in relationship to the neoplastic follicle. Follicular pattern was defined as a majority of cells localized to the follicle or perifollicular area, whereas the diffuse pattern a majority of positive cells were not confined to the follicle. . Quantity and intensity of IHC stain were analyzed using a 0–3 scale. Assessment of IHC was done independently by two physicians (JPS and JMJ) with >90% concordance. Because identifying a single antigen in isolation through IHC does not identify the underlying immune cell that is represented, co-expression of multiple antigens was visualized using a novel method devised by Glass and colleagues (26). The resulting images captured were overlaid and each antigen assigned a color using Adobe Photoshop CS2 (Adobe Systems, Inc.; San Jose, CA). To differentiate CD14+ cells from macrophages and follicular dendritic cells (FDC), slides initially positive for CD14 were co-stained with CD68 and CD163 as well as CD21 and CD23. To differentiate different PD1+ cells such as exhausted effector T-cells and T follicular helper cells (TFH) cells, PD1+ positive cells were co-stained with CD3, CD19, CXCR5 and TIM3.
Flow Cytometry
Cells were stained with fluorochrome conjugated antibodies to human ICAM (R&D Systems, Minneapolis, MN; clone BBIG-l1), CD3 (clone HIT3a), CD19 (clone 4G7), PD1 (clone EH12.1), CD14 (clone M5E2), CD163 (clone GH1/61), CD21 (clone B-ly4), CD273 (clone M1H18), CD274 (clone M1H1), TIM3 (clone 344823), and CD23 (clone EBVCS-5) (all obtained from BD Biosciences, San Jose, CA) and analyzed by flow cytometry and the data were analyzed using CellQuest software (Bectin Dickinson, San Jose CA).
Cell Culture
FDC enriched fractions were isolated as described in Schriever (27) et al with some modifications. Briefly, tonsils obtained from routine tonsillectomy were cut into small pieces and incubated for 1h at 37° with constant shaking in RPMI media containing 90ug/ml gentamicin , 2mg/ml collagenase (Worthington Biochemical, Lakewood, NJ) and 0.1mg/ml DNase (Sigma, St. Louis, MO). The digest was cooled on ice, filtered through a 70um nylon filter and centrifuged. The cell pellets were resuspended in RPMI, layered onto a discontinuous Percoll (Pharmacia, Uppsala, Sweden) gradient and centrifuged for 30 min at 1800 rpm. Cells at the 15%/35% interface were collected and washed. Cells were resuspended at a concentration of 4×106/ml and cultured in tissue culture dishes overnight. Non adherent cells were removed at 24 hours and fresh media added to the adherent cells. Adherent cells were trypsinized when confluent and analyzed for FDC markers by flow cytometry (FACS Caliber, Bectin Dickinson, San Jose, CA). CD19+ cells were isolated from lymphoma samples by positive selection and cultured with FDC cells at a ratio of 2.5:1. At 48h viability of CD19+ cells was determined by the percentage of B-cells that were negative by flow cytometry for Annexin V and PI.
Statistical analysis
Kaplan- Meier method was used to analyze time to transformation (TTT) (defined as time of diagnosis until transformation to DLBCL) and overall survival (OS) (defined as time of diagnosis until time of death). OS was censored at date of last follow up. Clinical characteristics including FLIPI score as well as IHC score and patterns were analyzed using log rank analysis for TTT and OS. Significant factors from univariate analysis were evaluated in a multivariate model using Cox proportional hazards regression. Statistical analysis utilized JMP 9.0.1 software (SAS Institute Inc., Cary, NC, USA).
Results
There were 58 patients with follicular lymphoma that later transformed to DLBCL. Patients presented with clinical symptoms compatible with transformation including rapidly progressive lymphadenopathy, increasing serum LDH or new constitutional symptoms. The diagnosis of transformation to large cell lymphoma however was confirmed by histological analysis in all cases. The median age at diagnosis was 64 years old and 24 (41%) were male. Other clinical baseline characteristics are summarized in Table 1. The majority (72%) of patients had advanced stage disease and all patients were either follicular grade 1 or 2 non-Hodgkin lymphoma. The FLIPI scores at diagnosis were: 18 (31%) low risk, 27 (47%) intermediate risk and 12 (21%) high risk. 42 (72%) patients were initially observed, 9 (15%) were treated with a combination of cyclophosphamide, vincristine and prednisone and 5 (9%) received an anthracycline-combination. Only 2 patients received rituximab. The median time to transformation (TTT) was 4.7 years (range, 0.4–20 years). The median estimated follow up for patients still alive (n=15) was 22.5 years while the median overall survival (OS) for entire cohort was 9.2 years. Gender, stage, absolute lymphocyte count, presence of constitutional symptoms and grade did not correlate to TTT or OS. The FLIPI score was predictive of the rate of transformation to DLBCL (p=0.01) and a lower FLIPI score was associated with improved median OS (p=0.04).
Table 1.
Patient Characteristics
| N=58 | % | |
|---|---|---|
| Gender | ||
| Female | 34 | 59 |
| Male | 24 | 41 |
| Age Median (range) | 64 (34–84) | |
| <60 years | 40 | 69 |
| >=60 years | 18 | 31 |
| Constitutional symptoms | ||
| Fever | 2 | 3 |
| Night Sweats | 2 | 3 |
| Weight loss | 1 | 2 |
| Stage | ||
| I | 7 | 12 |
| II | 9 | 15 |
| III | 8 | 14 |
| IV | 34 | 58 |
| Grade | ||
| 1 | 43 | 74 |
| 2 | 15 | 26 |
| FLIPI1 | ||
| 0 | 3 | 5 |
| 1 | 15 | 26 |
| 2 | 27 | 47 |
| 3 | 12 | 21 |
| ALC 2 | ||
| </= 1.0 | 31 | 69 |
| >1.0 | 14 | 31 |
Follicular lymphoma internation prognostic index,
absolute lymphocyte count
Relationship between CD14+ cells and time to transformation
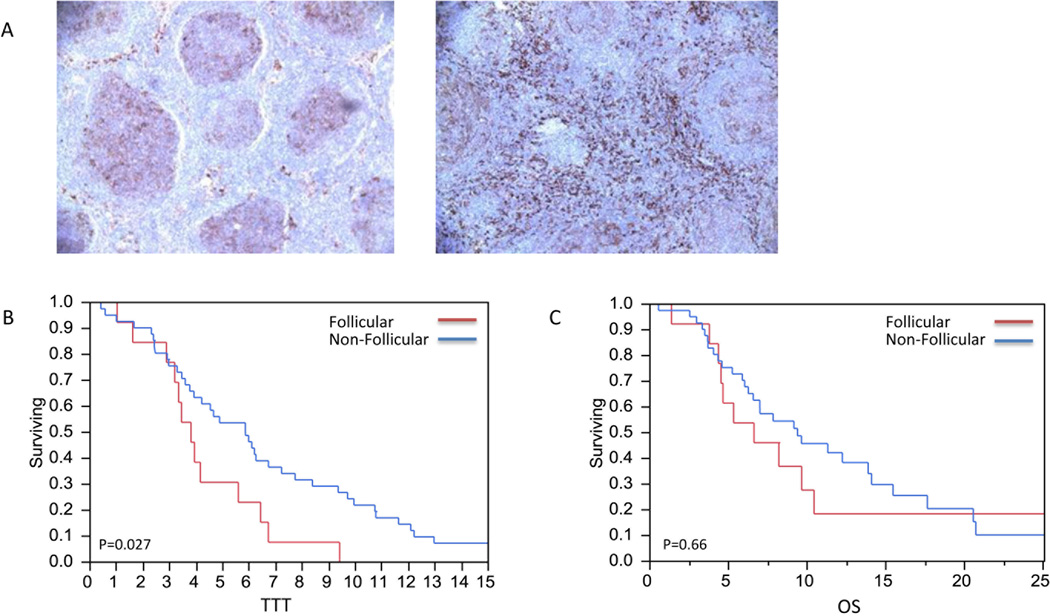
Paraffin tissue specimens were analyzed by pattern of location and cell content as described in the methods section. No relationship with TTT or OS was seen with the number or distribution of cells expressing FOXP3, CXCL3, CD21, CD68 or CD11c. The quantity of CD14+ cells was also not associated with TTT or OS; however, the location of CD14+ cells was predicative of time to transformation. As referenced in the methods, CD14+ cells were categorized based on pattern of location, follicular (n=13) and non-follicular (n=41) (Figure 1A). Patients with CD14+ cells localized to the follicle had a median TTT of 3.8 years compared to 5.9 years for those with a non-follicular staining pattern (p=0.027; Figure 1B). The location of CD14+ cells was not associated with a significant difference in OS (p= 0.66; Figure 1C).
Figure 1. CD14+ cells pattern of expression and TTT.
A) Follicular and non-follicular pattern of CD14+ staining. B) CD14+ cells localized to the follicle had a shorter time to transformation compared to those that were not localized to the follicle (2.8 vs 5.9 years, p=0.27). C) Follicular CD14+ cells were not associated with improvement in OS (p=0.66).
Relationship between PD1+ cells and time to transformation
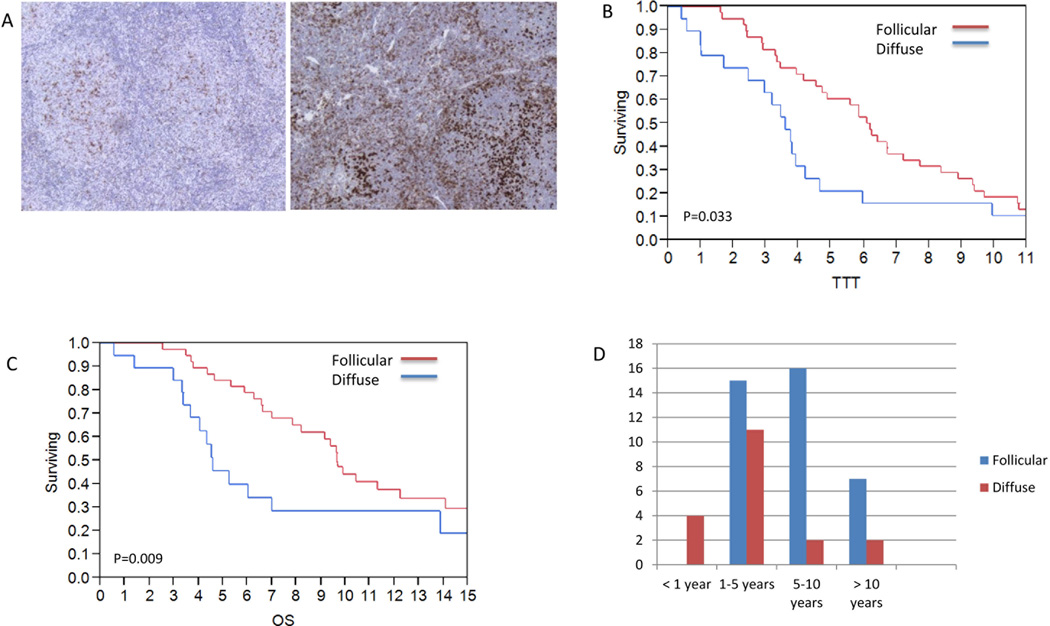
Similar to CD14+ cells, the relative quantity of PD1+ cells was not associated with either OS or TTT; however the location of PD1+ cell was predictive of clinical outcomes. 38 patients had a follicular pattern of PD1+ cells and 19 a diffuse pattern (Figure 2A). Patients with PD1+ cells localized to the follicle had a median TTT of 6.1 years compared to 3.6 years for those with a diffuse pattern (Figure 2B; p=0.033). The median OS for patients with PD1+ cells localized to the follicle was 9.7 years vs. 4.6 years for those with a diffuse distribution of PD1+ cells (Figure 2C; p=0.009). Categorizing the samples by early (<1 year) versus late (> 5 years) transformation confirmed that early transformation exclusively involved patients with a diffuse pattern of PD1+ cell expression, while late transformation predominantly involved patients with a follicular pattern (Figure 2D). Of note, the pattern of PD1+ and CD14+ cells did not correlate with each other (p=0.36). Instead, the localization of PD1+ and CD14+ appeared to have an inverse relationship, as patients with PD1+ cells localized to the follicle had superior outcomes while those with CD14+ cells localized to follicle had inferior outcomes. Patients with both follicular PD1+ and diffuse CD14+ cells had a delay in time to transformation (6.4 vs 3.2 yrs.) compared to patients with follicular CD14+ cells and diffuse PD1+ cells (p= 0.01). Previous studies have identified the FLIPI score, anemia and an elevated LDH as associated with transformation (28). The FLIPI score, which incorporates anemia and LDH, was included in a multivariate analysis and the pattern of PD1+ (HR=1.9, 95% CI 1.0–3.5, p=0.045) and CD14+ (HR=3.0, 95% CI 1.5–6.1, p=0.004) cells remained significantly associated with inferior TTT. After accounting for the FLIPI score, the diffuse pattern of PD1+ cells remained significantly associated with an inferior OS (HR=2.5, 95% CI 1.2–4.8, p=0.012) while the pattern of CD14 cells was not (HR=1.4 95% CI 0.6–2.9, p=0.37).
Figure 2. PD1+ cell pattern and TTT and OS.
A) Follicular and diffuse pattern of PD1+ cell expression. Follicular pattern of PD1+ cells is associated with (B) prolonged time to transformation (6.1 vs.3.6 year, p=0.03) and (C) overall survival (9.7 vs 4.6 years, p=0.009) compared with diffuse pattern. D) All patients with early transformation (<1 yr.) had diffuse staining of PD1+ cells compared to follicular pattern predominating amongst late transformation (>5 yrs.)
Identification of PD1+ Immune cells
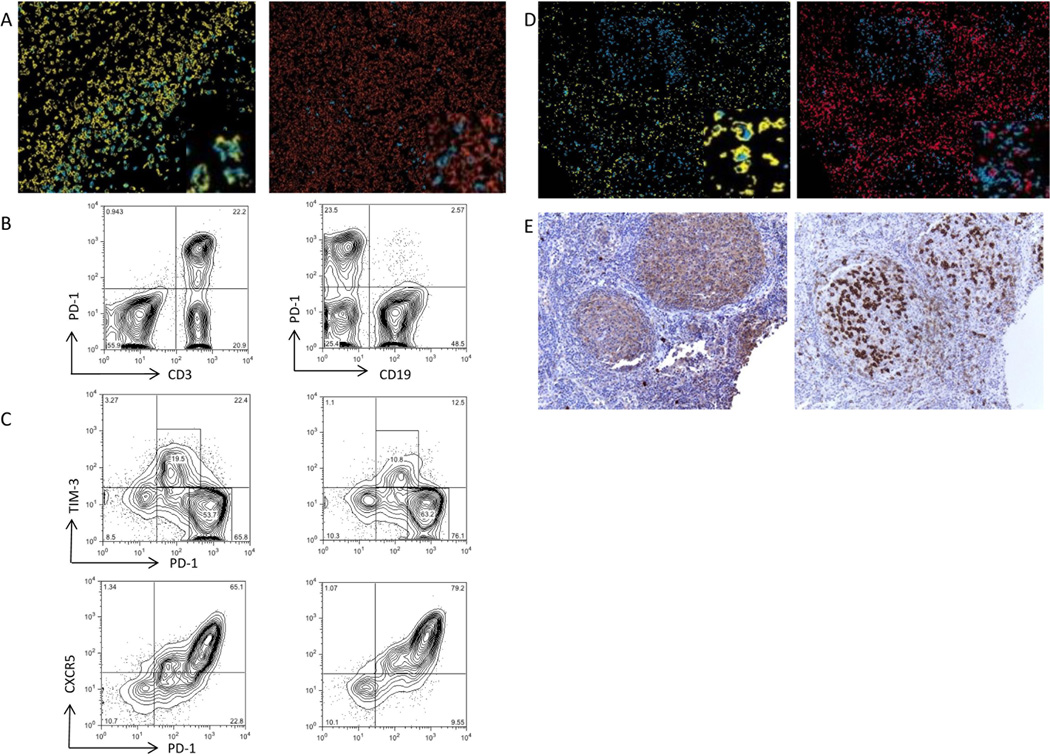
As different immune cells can express PD1 when evaluated by IHC, one cannot reliably identify the underlying immune cell of interest by using single IHC staining. Multicolor IHC was therefore undertaken to better identify which immune cells are represented by PD1+ staining. PD1+ cells are thought to be primarily T cells but it can also be expressed on activated B-cells (29). Multicolor IHC illustrated that PD1+ cells in our sample primarily expressed CD3 and did not co-localize with the B-cell marker CD19 (Figure 3A). To further analyze other markers of co-expression, flow cytometry was performed on follicular lymphoma samples with antibodies to CD3, CD19 and PD1. Again, CD3 was predominantly co-expressed with PD1 (Figure 3B) with low levels of PD1 expression on B-cells that was similar when compared to normal hyperplastic tonsils (data not shown).
Figure 3. PD1+ cells co-expression.
A) Multicolor IHC with magnified inserts of CD3 (yellow) and PD1 (blue) demonstrating co-localization of staining while CD 20 (orange ) is not co-localized to PD1+ cells. B) Flow cytometry demonstrates primary co-expression with PD 1 and CD3 compared to PD1 and CD19 (n=5). C) Flow cytometry of patient samples illustrates PD1+ cells co-express CXCR5 and TIM3 (n=3). D) Multicolor IHC with magnified inserts confirms that PD1+ (blue) cells in follicle are positive CD3 (pink) and negative for TIM3 (yellow), while PD1 cells outside follicle are positive for TIM3. . E) CXCR5 and PD1 both localize to the follicle suggesting coexpression.
Previous studies have identified PD1 on T follicular helper (TFH)cells (30). TFH cells express CXCR5 and we and others have previously demonstrated that PD1+ cells in follicular lymphoma express CXCR5 (31, 32) . However, given the divergent outcomes based on the pattern of localization of PD1+ cells we wondered whether these different areas (follicular vs interfollicular) represented two separate populations. In addition to TFH, PD1 expression has also been described on exhausted T cells (32). As these exhausted cells characteristically express TIM3, this can act as a marker for the presence of exhausted T-cells(32). Flow cytometry on follicular samples confirmed co-expression of CXCR5 and PD1 and also identified a population of PD1+ cells that coexpress TIM3 (Figure3C). There was little TIM3 expression on cells outside of the lymphocyte gate suggesting that TIM3 is not expressed on other immune subsets such as dendritic cells. Multicolor IHC with CD3, PD1 and TIM3 confirmed that PD1+ cells in the follicle were positive for CD3 but completely negative for TIM3; however, PD1+ cells in the interfollicular space did co-localize with TIM3 (Figure 3D). Cells in the follicle stained positive for both CXCR5 and PD1 (Figure 3E). These results confirm that PD1+ cells in the follicle are TIM3 negative and do not represent exhausted T cells, and given their location and the findings of IHC and flow cytometry it appears that these cells are primarily TFH cells. Thus the improved clinical outcomes associated with the follicular pattern of PD1+ cells such as delay in time to transformation and improved survival are related to the maintained presence of TFH cells. PD1+ cells outside of the follicle are a distinct cell type compared to follicular PD1+ cells. These PD1+TIM3+ cells that are outside the follicle appear to represent exhausted T cells and are associated with inferior clinical outcomes.
Identification of CD14+ cells
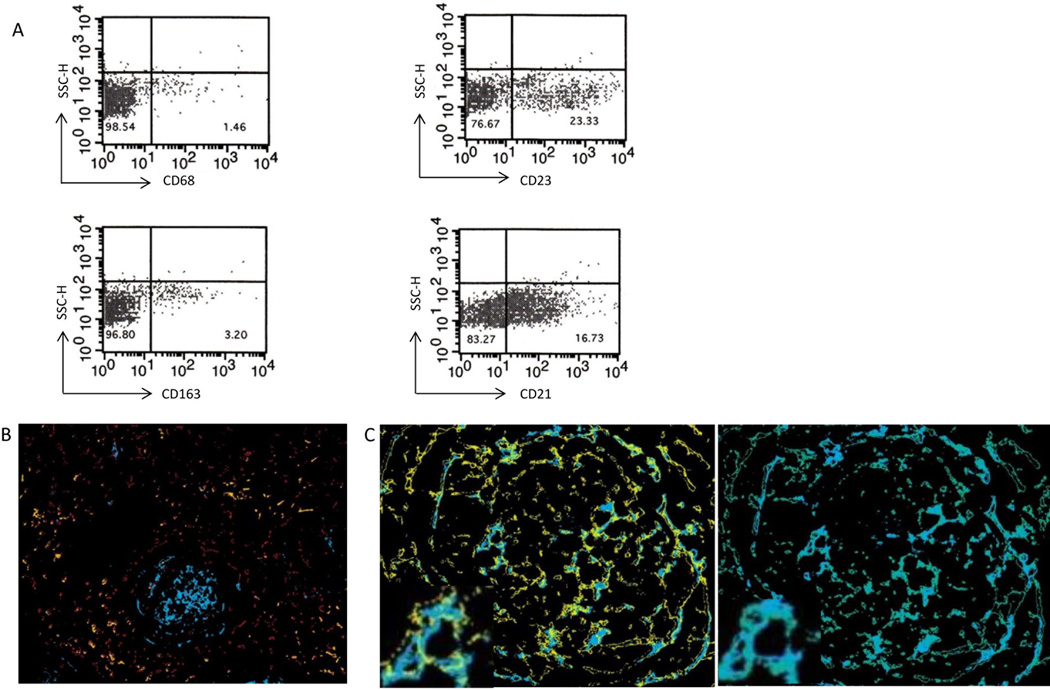
CD14 is often associated with monocytes, however CD14 is not exclusively expressed on monocytic cells and can be seen on other cell types (33). We analyzed our CD14+ cell population using multicolor flow cytometry and IHC to various monocytic, macrophage and dendritic cell markers to better characterize the underlying cell type. Flow cytometry demonstrated minimal co-expression of CD14 with either CD68 or CD163 (Figure 4A). However, a substantial portion of CD14+ cells co-expressed CD21 and CD23, which are markers typically associated with follicular dendritic cells (FDC). Multicolor IHC demonstrated that CD21 and CD23 were co-expressed on nearly all CD14+ cells and were absent on CD68+ and CD163+ cells (Figures 4B and C). These results confirm the flow cytometry findings and suggest that rather than representing monocytes or macrophages, these CD14+ cells are FDC.
Figure 4. CD14+ cells consistent with FDC.
A) Flow cytometry demonstrates coexpression primarily on CD21 and 23 and not CD68 and 163 (n=3). B) Multicolor IHC of CD14 (blue), CD68 (red) and CD163 (orange) demonstrating no co-localization. (C) Cells staining for CD 14 (blue) co-localize to identical cells staining positive for CD21 (yellow) and CD 23 (green) exemplified on magnified inserts.
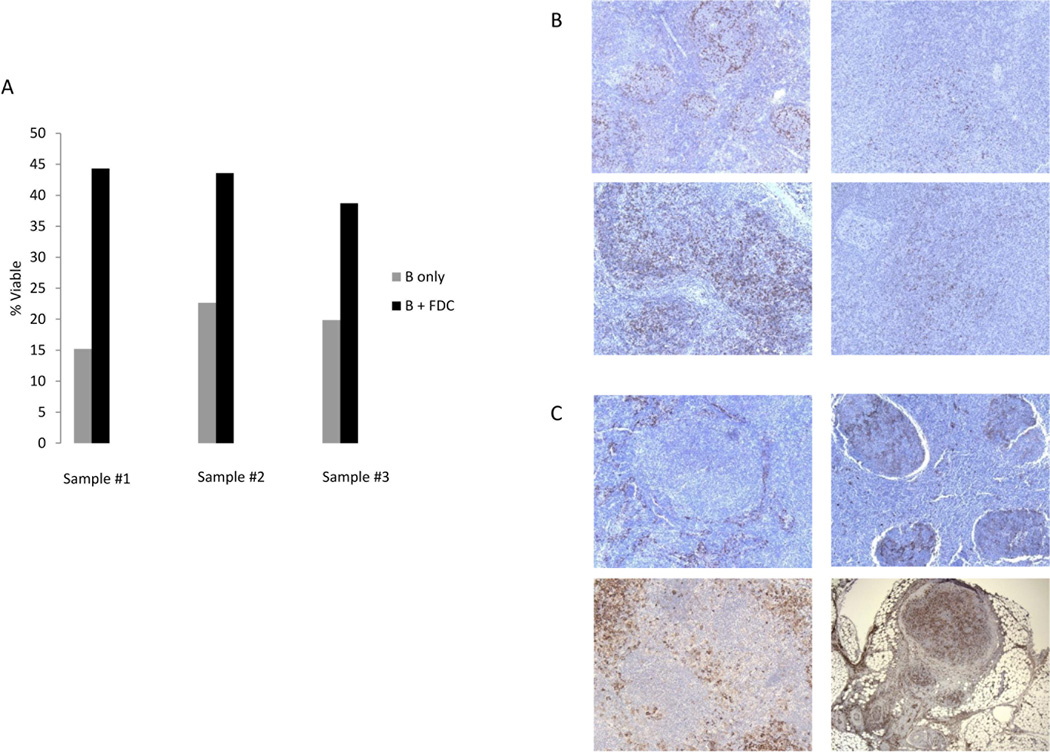
Follicular dendritic cells promote malignant cell viability
The prognostic role of FDC in follicular lymphoma is unclear. Our results suggest an association of inferior outcomes with an increase in CD14+ cells that localize to the follicle and whose immunophenotype is consistent with FDC. To understand how increased follicular dendritic cells affect follicular lymphoma, we co-cultured B-cells from follicular lymphoma patients with FDC and assessed for changes in cell viability compared to untreated follicular lymphoma B-cells. After 48 hours, the cell viability of B cells doubled, 42% (± 1.7) vs. 19% (±2.2), with the presence of FDC compared to controls (Figure 5A; p=0.003). These results demonstrate that FDC prolong the survival of B cells in follicular lymphoma. Given their shared localization the neoplastic follicle, CD14+ FDC may prolong survival of the tumor cells in follicular lymphoma. Though promotion of viability is not directly related to transformation, any mechanism that promotes malignant B-cell survival may well promote transformation. Because it is difficult to use fresh patient tissue to show proliferation or intracellular signaling, we chose promotion of malignant B-cell viability as evidence that FDC support malignant B-cell growth.
Figure 5. FDC increase CD19 + follicular lymphoma cell survival.
FDC were co-cultured with follicular lymphoma patient samples using positively selected CD19+ cells at a ratio of 2.5:1. After 48 hours the viability of the CD19+ follicular lymphoma cells was assessed by Annexin V/PI. The presence of FDC was associated with significant increase in viability of CD19+ cells (p=0.003). B) Sequential biopsies from diagnosis to transformation from two representative patient samples demonstrate a transition from PD1+ cells in the follicle to a diffuse pattern as patient nears transformation. C) CD14+ cells change across time from non-follicular to follicular pattern.
Sequential biopsies
A potential limitation of our study is the long interval between the immunological findings on diagnostic biopsy and the date of transformation in some of the patients. It could be argued that the biopsies were taken at a fixed time point (diagnosis), but that the changes in the microenvironment continue to evolve over time. To address this further, we analyzed CD14+ and PD1+ cell expression on samples from 6 patients where serial biopsies were available from diagnosis to transformation. On average there were 3 serial biopsies per patient, the pathology varied from hyperplasia to follicular lymphoma and the time range encompassed was from 3–16 years. The intensity of PD1 or CD14 cell staining did not change in serial biopsies as patients progressed to transformation. However, serial biopsies confirmed that the pattern of CD14+ cells transitioned from a non-follicular to follicular pattern as the time of transformation approached. Similarly, the pattern of PD1 expression changed from a follicular to a diffuse pattern prior to transformation (Figure 5B, C). These serial biopsies demonstrate an evolution in the pattern of CD14 and PD1 expression as patients approach transformation.
Discussion
This study demonstrates that the pattern of CD14+ and PD1+ cells are predictive of the time to transformation in follicular lymphoma patients. Patient’s samples where CD14+ cells localized to the follicle were associated with a shorter time to transformation than those that did not. Conversely, patient samples where PD1+ cells were no longer localized to the follicle were associated with a shorter time to transformation and inferior survival. These results were independent of each other and remained statistically significant after accounting for clinical factors such as the FLIPI score.
This is the first report of an association of CD14+ cells predicting outcomes in patients with follicular lymphoma. Rather than the quantity of CD14+ cells, it was their location within the tumor and microenvironment that was predictive of outcome. CD14 is a lipopolysaccharide binding protein that can act as receptor for endotoxin that is often associated with monocytes (34). Lymphoma associated monocytes have been associated with poor survival in follicular lymphoma(18, 35) and initially it was assumed the CD14+ cells represented intratumoral monocytes. However, further analysis through flow cytometry and multicolor IHC confirmed that the CD14+ intrafollicular cells were distinct from monocytes or macrophages and represented FDC. Though FDC are typically identified by CD21, CD23 and CD35 surface markers they have also previously been associated with CD14 as well (36). CD14+ monocytes are thought to be immunosuppressive and their presence is associated with more aggressive tumors in NHL (24); however the role of CD14+ cells that are representative of a FDC phenotype is unknown.
Previous gene expression profiles of follicular lymphoma patients identified that an increase in genes expressed by dendritic cells was associated with poor overall survival (11). A recent study assessed the prognostic role of FDC in follicular lymphoma based on the extent of follicular dendritic cells(37). They found no association with clinical outcomes; however, this study focused on all FDC using a pan-FDC antibody where as in our study the predictive component was limited to CD14+ cells. Similarly, in this current study no association with clinical outcomes was seen when the more common FDC marker CD21+ was compared, this suggests that the CD14+ FDC of interest may represent a unique subset of FDC. To clarify the role of FDC in follicular lymphoma we co-cultured FDC and malignant B –cells and demonstrated the presence of FDC cells led to increased B-cell viability. Others have also found that FDC promote the proliferation and prevent apoptosis of transformed malignant B-cells (38, 39). Though there are limitations in these studies as tonsil derived FDC were utilized and viability rather than transformation was measured, these results confirm these previous findings and provide a biologic rationale for the observation that CD14+ FDC localized to the follicle are associated with a shorter time to transformation.
In addition to CD14+ FDC, PD1+ cells were found to be strongly correlated with clinical outcomes. Patients whose biopsies demonstrated PD1+ cells that localized to the follicle had a significantly prolonged time to transformation and overall survival compared to those with a diffuse pattern of PD1+ cells. The role of PD1 in solid tumors is well recognized with some solid tumors expressing PD-L1 and this ligand for PD1 being associated with a poor prognosis possibly through a decrease in tumor immune surveillance(40, 41). Previous analyses of PD1+ cells in follicular lymphoma have yielded conflicting results. Two studies supported that increased levels of PD1+ cells were associated with superior outcomes including a decreased risk of transformation(13, 25). However, additional studies have not confirmed the correlation between improved outcome and an increased number of PD1+ cells (42). In fact, some studies have found that increased PD1+ cells are associated with an inferior survival in follicular lymphoma (15). Though this study found no association between the TTT and the quantity of PD1+ cell content, it, like other microenvironment analyses, found an association between the location within the microenvironment and patient outcomes (14, 17, 43). When reviewing the previous analyses that did find a positive association of PD1+ cells and survival, in one study >90% of PD1+ cells were localized to the follicle and in the other the results were accentuated when the analysis was limited to the follicular subset (25). Therefore our observation of superior clinical outcomes when PD1+ cells are localized to the follicle appears to be in agreement with these previous studies.
The retrospective and subjective nature of all of these studies, this study included, can affect their reproducibility and concordances with other studies. Additionally, analysis restricted to single marker by IHC may identify a heterogenous population of different underlying immune cells that share a similar marker. To clarify this, multicolor IHC and flow cytometry were utilized in this study to identify PD1+ cells and showed that PD1+ cells in the follicle are primarily TFH cells while those that are outside of the follicle are TIM3+ exhausted T-cells. These results are consistent with other findings that PD1 is expressed on TFH cells, however, they also illustrate that not all PD1+ cells are the same. The prognosis of TFH cells in follicular lymphoma is currently unknown. However, our results suggest that superior clinical outcomes including a prolonged time to transformation that are associated with follicular PD1+ cells are attributable to TFH cells. In contrast, we have previously demonstrated that exhausted T-cells identified as TIM3+ cells are associated with inferior prognosis in follicular lymphoma(32). This study demonstrates that the PD1+ cells that reside outside the follicle represent TIM3+ exhausted T-cells and the presence of these PD1+TIM3+ cells is associated with poor survival and shorter time to transformation. One possible explantation is that a subset of T-cells function to suppress transformation and with T-cell exhaustion this suppression is lost thereby allowing for transformation.
This study did not find an association with increased quantity of CD68+ macrophages or FOXP3+ cells and clinical outcomes as others have previously reported(12, 18). This could be related to sample size, variation in treatment received as well as difference in technique and interpretation of IHC. However, follow up studies of the significance of increased CD68+ macrophages have been contradictory(19, 43, 44). Similarly, further studies have not confirmed the association of increased FOXP3+ cells and superior outcomes in follicular lymphoma(17, 44); in fact, Carreras et al noted that after adjusting for the presence of PD1+ cells, FOXP3 was no longer associated with improved survival(13). In addition, this study involved a majority of patients treated prior to the rituximab era. Thus, our microenvironment findings do not reflect the potential effects of rituximab treatment and previous studies have demonstrated that the introduction of rituximab can affect microenvironment associations(19). Furthermore, previous studies have documented a constant annual rate of transformation rather than a dynamic rate of transformation, as seen in this study, which may suggest an evolving change in the microenvironment . The microenvironment factors identified in our analysis therefore likely contribute to transformation but may not be comprehensive. It is conceivable that the interactions and effects of PD1 and CD14 cells with other immune cells and/or the tumor itself lead to a more stepwise development of transformation.
This study identifies two independent factors of the follicular lymphoma microenvironment that are associated with a shorter time to transformation. In both instances it is the pattern of location rather than quantity of PD1+ and CD14+ cells that is predictive of clinical outcomes. This study is the first to report an association between CD14+ FDC and clinical outcomes in follicular lymphoma. The study also provides additional understanding to the complex interaction of PD1 in follicular lymphoma. We find that PD1+ TFH cells are associated with superior clinical outcomes, while PD1+TIM3+ exhausted T cells outside the follicle are associated with an inferior survival and a shorter time to transformation. Additional prospective studies are warranted to confirm these findings and determine their prognostic importance relative to other previously identified predictive factors in the microenvironment. Further understanding of these interactions could be useful in designing therapies that modulate the immune microenvironment in follicular lymphoma.
Translational Relevance.
This study identifies two independent architectural biomarkers associated with a shorter time to transformation in follicular lymphoma. CD14+ intratumoral cells localized to the follicle were associated with a shorter time to transformation; whereas, PD1+ cells present in a diffuse pattern had a shorter time to transformation compared to a follicular pattern. Multicolor immunohistochemistry and flow cytometry identified CD14+ cells as follicular dendritic cells (FDC). These FDC were shown to promote B cell growth. Two separate populations of PD1+ cells were identified; with PD1+/TIM3+ exhausted T cells associated with an inferior survival and shorter time to transformation. This is the first study to report an association of intratumoral CD14+ cells and clinical outcomes in follicular lymphoma. In addition, it provides additional understanding of the various PD1+ subsets found throughout the microenvironment. Overall these results further the understanding of the tumor microenvironment and will aid in the design and use of immunotherapies.
Acknowledgments
Supported in part by grants CA92104 and CA97274 from the National Institutes of Health, the Lymphoma Research Foundation, the Leukemia & Lymphoma Society and the Predolin Foundation.
Footnotes
Conflict of Interests Disclosures: None of the authors have any conflicts of interest to disclose.
Authorship Contributions: JPS preformed research, analyzed data, preformed statistical analysis and wrote the manuscript. JMJ, SZ, DG, BX, KR and ALF performed research and collected data. GSN, JRC and AJN designed the study and contributed to writing the manuscript. SMA designed the study, analyzed data and wrote the manuscript.
References
- 1.Al-Tourah AJ, Gill KK, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:5165–5169. doi: 10.1200/JCO.2008.16.0283. [DOI] [PubMed] [Google Scholar]
- 2.Bastion Y, Sebban C, Berger F, Felman P, Salles G, Dumontet C, et al. Incidence, predictive factors, and outcome of lymphoma transformation in follicular lymphoma patients. J Clin Oncol. 1997;15:1587–1594. doi: 10.1200/JCO.1997.15.4.1587. [DOI] [PubMed] [Google Scholar]
- 3.Gine E, Montoto S, Bosch F, Arenillas L, Mercadal S, Villamor N, et al. The Follicular Lymphoma International Prognostic Index (FLIPI) and the histological subtype are the most important factors to predict histological transformation in follicular lymphoma. Ann Oncol. 2006;17:1539–1545. doi: 10.1093/annonc/mdl162. [DOI] [PubMed] [Google Scholar]
- 4.Montoto S, Davies AJ, Matthews J, Calaminici M, Norton AJ, Amess J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007;25:2426–2433. doi: 10.1200/JCO.2006.09.3260. [DOI] [PubMed] [Google Scholar]
- 5.Acker B, Hoppe RT, Colby TV, Cox RS, Kaplan HS, Rosenberg SA. Histologic conversion in the non-Hodgkin's lymphomas. J Clin Oncol. 1983;1:11–16. doi: 10.1200/JCO.1983.1.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Conconi A, Ponzio C, Lobetti-Bodoni C, Motta M, Rancoita PMV, Stathis A, et al. Incidence, risk factors and outcome of histological transformation in follicular lymphoma. Br J Haematol. 2012;157:188–196. doi: 10.1111/j.1365-2141.2012.09054.x. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher CJ, Gregory WM, Jones AE, Stansfeld AG, Richards MA, Dhaliwal HS, et al. Follicular lymphoma: prognostic factors for response and survival. J Clin Oncol. 1986;4:1470–1480. doi: 10.1200/JCO.1986.4.10.1470. [DOI] [PubMed] [Google Scholar]
- 8.Morley NJ, Evans LS, Goepel J, Hancock BW. Transformed follicular lymphoma: the 25-year experience of a UK provincial lymphoma treatment centre. Oncol Rep. 2008;20:953–956. doi: 10.3892/or_00000095. [DOI] [PubMed] [Google Scholar]
- 9.Solal-Celigny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 10.Yang ZZ, Ansell SM. The tumor microenvironment in follicular lymphoma. Clin Adv Hematol Oncol. 2012;10:810–818. [PubMed] [Google Scholar]
- 11.Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 12.Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108:2957–2964. doi: 10.1182/blood-2006-04-018218. [DOI] [PubMed] [Google Scholar]
- 13.Carreras J, Lopez-Guillermo A, Roncador G, Villamor N, Colomo L, Martinez A, et al. High Numbers of Tumor-Infiltrating Programmed Cell Death 1–Positive Regulatory Lymphocytes Are Associated With Improved Overall Survival in Follicular Lymphoma. Journal of Clinical Oncology. 2009;27:1470–1476. doi: 10.1200/JCO.2008.18.0513. [DOI] [PubMed] [Google Scholar]
- 14.Lee AM, Clear AJ, Calaminici M, Davies AJ, Jordan S, MacDougall F, et al. Number of CD4+ Cells and Location of Forkhead Box Protein P3–Positive Cells in Diagnostic Follicular Lymphoma Tissue Microarrays Correlates With Outcome. Journal of Clinical Oncology. 2006;24:5052–5059. doi: 10.1200/JCO.2006.06.4642. [DOI] [PubMed] [Google Scholar]
- 15.Richendollar BG, Pohlman B, Elson P, Hsi ED. Follicular programmed death 1–positive lymphocytes in the tumor microenvironment are an independent prognostic factor in follicular lymphoma. Human Pathology. 2011;42:552–557. doi: 10.1016/j.humpath.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Wahlin BE, Sander B, Christensson B, Kimby E. CD8+ T-Cell Content in Diagnostic Lymph Nodes Measured by Flow Cytometry Is a Predictor of Survival in Follicular Lymphoma. Clinical Cancer Research. 2007;13:388–397. doi: 10.1158/1078-0432.CCR-06-1734. [DOI] [PubMed] [Google Scholar]
- 17.Farinha P, Al-Tourah A, Gill K, Klasa R, Connors JM, Gascoyne RD. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood. 2010;115:289–295. doi: 10.1182/blood-2009-07-235598. [DOI] [PubMed] [Google Scholar]
- 18.Farinha P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, Gill K, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–2174. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 19.Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchhauser F, et al. High Numbers of Tumor-Associated Macrophages Have an Adverse Prognostic Value That Can Be Circumvented by Rituximab in Patients With Follicular Lymphoma Enrolled Onto the GELA-GOELAMS FL-2000 Trial. Journal of Clinical Oncology. 2008;26:440–446. doi: 10.1200/JCO.2007.12.8298. [DOI] [PubMed] [Google Scholar]
- 20.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 21.Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Gastineau DA, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12:631–644. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lekkou A, Karakantza M, Mouzaki A, Kalfarentzos F, Gogos CA. Cytokine production and monocyte HLA-DR expression as predictors of outcome for patients with community-acquired severe infections. Clin Diagn Lab Immunol. 2004;11:161–167. doi: 10.1128/CDLI.11.1.161-167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, et al. Immunosuppressive CD14+HLA−DRlow/− monocytes in prostate cancer. Prostate. 2010;70:443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA−DR(low)/− monocytes in B-cell non-Hodgkin lymphoma. Blood. 2011;117:872–881. doi: 10.1182/blood-2010-05-283820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahlin BE, Aggarwal M, Montes-Moreno S, Gonzalez LF, Roncador G, Sanchez-Verde L, et al. A Unifying Microenvironment Model in Follicular Lymphoma: Outcome Is Predicted by Programmed Death-1–Positive, Regulatory, Cytotoxic, and Helper T Cells and Macrophages. Clinical Cancer Research. 2010;16:637–650. doi: 10.1158/1078-0432.CCR-09-2487. [DOI] [PubMed] [Google Scholar]
- 26.Glass G, Papin JA, Mandell JW. SIMPLE: a sequential immunoperoxidase labeling and erasing method. J Histochem Cytochem. 2009;57:899–905. doi: 10.1369/jhc.2009.953612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schriever F, Freedman AS, Freeman G, Messner E, Lee G, Daley J, et al. Isolated human follicular dendritic cells display a unique antigenic phenotype. J Exp Med. 1989;169:2043–2058. doi: 10.1084/jem.169.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Link BK, Maurer MJ, Nowakowski GS, Ansell SM, Macon WR, Syrbu SI, et al. Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: a report from the University of Iowa/MayoClinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J Clin Oncol. 2013;31:3272–3278. doi: 10.1200/JCO.2012.48.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 30.Crotty S. Follicular Helper CD4 T Cells (TFH) Annual Review of Immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 31.Ame-Thomas P, Le Priol J, Yssel H, Caron G, Pangault C, Jean R, et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia. 2012;26:1053–1063. doi: 10.1038/leu.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, et al. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest. 2012;122:1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jersmann HPA. Time to abandon dogma: CD14 is expressed by non-myeloid lineage cells. Immunol Cell Biol. 2005;83:462–467. doi: 10.1111/j.1440-1711.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- 34.Naeim F, Rao PN, Grody WW. Hematopathology: Morphology, Immunophenotype, Cytogenetics, and Molecular Approaches. Elsevier Science; 2009. [Google Scholar]
- 35.Kridel R, Tan K, Al-Tourah AJ, Moccia AA, Scott DW, Slack GW, et al. Tumor-Associated Macrophages Predict Outcome in Follicular Lymphoma. ASH Annual Meeting Abstracts. 2012;120 682-. [Google Scholar]
- 36.Petrasch S, Perez-alvarez C, Schmitz J, Kosco M, Brittinger G. Antigenic phenotyping of human follicular dendritic cells isolated from nonmalignant and malignant lymphatic tissue. Eur J Immunol. 1990;20:1013–1018. doi: 10.1002/eji.1830200510. [DOI] [PubMed] [Google Scholar]
- 37.Jin MK, Hoster E, Dreyling M, Unterhalt M, Hiddemann W, Klapper W. Follicular dendritic cells in follicular lymphoma and types of non-Hodgkin lymphoma show reduced expression of CD23, CD35 and CD54 but no association with clinical outcome. Histopathology. 2011;58:586–592. doi: 10.1111/j.1365-2559.2011.03779.x. [DOI] [PubMed] [Google Scholar]
- 38.Park CS, Choi YS. How do follicular dendritic cells interact intimately with B cells in the germinal centre? Immunology. 2005;114:2–10. doi: 10.1111/j.1365-2567.2004.02075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goval JJ, Thielen C, Bourguignon C, Greimers R, Dejardin E, Choi YS, et al. The prevention of spontaneous apoptosis of follicular lymphoma B cells by a follicular dendritic cell line: involvement of caspase-3, caspase-8 and c-FLIP. Haematologica. 2008;93:1169–1177. doi: 10.3324/haematol.12127. [DOI] [PubMed] [Google Scholar]
- 40.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 41.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 42.Farinha P, Roncador G, Al-Tourah A, Connors JM, Gascoyne RD. Combined FOXP3+ and PD1+ T cell density and architectural patterns predict overall survival and risk of transformation in uniformly treated patients with follicular lymphoma. Blood (ASH Annual Meeting Abstracts) 2008;112:2815. [Google Scholar]
- 43.Glas AM, Knoops L, Delahaye L, Kersten MJ, Kibbelaar RE, Wessels LA, et al. Gene-expression and immunohistochemical study of specific T-cell subsets and accessory cell types in the transformation and prognosis of follicular lymphoma. J Clin Oncol. 2007;25:390–398. doi: 10.1200/JCO.2006.06.1648. [DOI] [PubMed] [Google Scholar]
- 44.Sweetenham JW, Goldman B, LeBlanc ML, Cook JR, Tubbs RR, Press OW, et al. Prognostic value of regulatory T cells, lymphoma-associated macrophages, and MUM-1 expression in follicular lymphoma treated before and after the introduction of monoclonal antibody therapy: a Southwest Oncology Group Study. Ann Oncol. 2010;21:1196–1202. doi: 10.1093/annonc/mdp460. [DOI] [PMC free article] [PubMed] [Google Scholar]