Abstract
Various local drug delivery devices and coatings are being developed as slow, sustained release mechanism for drugs, yet the polymers are typically not evaluated after commercial sterilization techniques. We examine the effect that commercial sterilization techniques have on the physical, mechanical, and drug delivery properties of polyurethane polymers. Specifically we tested cyclodextrin-hexamethyl diisocyanate crosslinked polymers before and after autoclave, ethylene oxide, and gamma radiation sterilization processes. We found that there is no significant change in the properties of polymers sterilized by ethylene oxide and gamma radiation compared to non-sterilized polymers. Polymers sterilized by autoclave showed increased tensile strength (p<0.0001) compared to non-sterilized polymers . In the release of drugs, which were loaded after the autoclave sterilization process, we observed a prolonged release (p<0.05) and a prolonged therapeutic effect (p<0.05) but less drug loading (p<0.0001) compared to non-sterilized polymers. The change in the release profile and tensile strength in polymers sterilized by autoclave was interpreted as being caused by additional crosslinking from residual, unreacted, or partially-reacted crosslinker contained within the polymer. Autoclaving therefore represents additional thermo-processing to modify rate and dose from polyurethanes and other materials.
Introduction
Polymers are widely used as biomedical implants and devices, yet changes in polymer properties after sterilization remain a prevalent problem in the development of new biopolymers. Sterilization of all medical implants prior to implantation is important,1 and sterilization procedures can have a significant effect on the polymers’ properties, including the release rate of an implantable drug delivery system.2, 3
The sterilization of a polymer is considered to be significantly challenging, and the sterilization protocol for a drug delivery polymer needs to be evaluated prior to any clinical trials.4, 5 In general, polymers can degrade or decompose when exposed to the kinetic energy of sterilization techniques;6-8 in addition the sterilization procedures can further crosslink materials as well.3, 9, 10 The changes caused by sterilization are especially prevalent in hydrogels; the embedded water content of hydrogels aids in the breakdown of chemical bonds during the sterilization procedure.11,12 To avoid the changes that occur during sterilization, alternative sterilization techniques are used, such as UV sterilization or ethanol soaking, yet the alternative techniques are challenging to precisely replicate, are not industrial standards, and may not fully sterilize the biomaterial.13-15 Therefore, it is still best to evaluate novel biomaterials with traditional sterilization techniques.6, 14, 16
The four most common sterilization procedures for drug delivery polymers are dry heat, autoclave, ethylene oxide, and gamma radiation. Each of these common sterilization techniques causes different problems in polymer crosslinked materials. Dry heat sterilization, which is the most basic sterilization procedure, heats the material at 170°C for over 1 hr, which will coagulate proteins and thus destroy any microorganisms present.17 Because of this high temperature, most polymers would soften or melt, and may also begin to degrade, leading to compromised thermomechanical properties and potentially altering the drug release profile of the non-sterilized material.6 Autoclave sterilization, which is the process of exposing the polymer to pressurized steam at 121°C, causes microorganisms to break down in the heated steam environment. Typically, plastics are not sterilized with autoclaving because the autoclave conditions can cause heat-sensitive materials to break down or melt.18 The degradation of the polymer typically leads to less drug loading or faster release.19 A third sterilization method is to expose the biomaterial to an extremely reactive gas, ethylene oxide, typically at 60°C. Though hot ethylene oxide has the ability to kill all known viruses and spores, remaining ethylene oxide in the polymer is extremely toxic and carcinogenic to the patient. To remove any remaining residual gas, a long degassing protocol is required, which may be challenging for highly porous or complex material geometries.20 In addition, ethylene oxide also has the ability to alter a polymer’s chemical structure.8, 20 Nevertheless, ethylene oxide sterilization remains the preferred method because it is the least likely to alter the drug delivery kinetics.21 Lastly, sterilization by gamma radiation is an increasingly popular technique because it has the ability for good penetration of the polymer, due to their low atomic density, without leaving any residual chemicals or toxins.22 Typically, the material is exposed to 25 kGy of radiation from a Colbalt-60 (60Co) source; however, the amount of radiation can be varied greatly depending on the biomaterial.15, 23 The high energy of gamma radiation has the ability to create and break bonds; most notably, it reduces crosslinking in orthopaedic crosslinked materials and some drug delivery polymers.7, 24-26 However, if needed, gamma radiation can also be intentionally used as a crosslinking agent, providing the energy needed to create additional crosslink bonds.3, 9
In our research we have explored polyurethane polymers, specifically the use of cyclodextrin polymers crosslinked with hexamethyldiisocyanate (HDI) as implantable drug delivery devices.27-31 These polymers have shown sustainable, long-term release of drugs in both in vitro and in vivo applications; however, we have not previously reported their performance after using commercial sterilization techniques. In an effort to understand how these polymers would interact with the various types of sterilization, the mechanical properties and drug release profiles were evaluated before and after the sterilization of each polymer.
Previously, diisocyanate generated polyurethanes have been evaluated after different sterilization techniques to determine the release of any broken linkages, specifically the leaching of 4,4′-methylenedianiline (MDA) as a result of sterilization.32 The conclusions of the MDA release were found to be inconclusive. Polymers sterilized with ethylene oxide sterilization did not show any changes in the MDA release. Polymers sterilized with autoclave or gamma radiation had an increase in leachants, and the quantity of MDA released was dependent on the monomers used and fabrication techniques. Still, the influence of sterilization on the thermomechanical properties and drug delivery from cyclodextrin-HDI polymers has never been previously evaluated. The presented results in thermomechanical property and drug delivery changes can be expanded to any biomaterial using diisocyanate as a crosslinker.
Experimental
Materials
β-cyclodextrin (CD, 2-15 kDa, average 10 CDs per chain) was purchased from CycloLab Ltd, (Hungry). CD was dried at 90°C for 24 hrs. Hexamethylene diisocyanate (HDI, Sigma Aldrich), rifampicin (Fisher Scientific), vancomycin hydrochloride, (MP Biomedicals, Inc), erythromycin (Sigma Aldrich), N,N-dimethylformamide (DMF, extra dry, Applied Biosystems) were used as received.
Polymer Fabrication and Cleaning
1.5 grams of CD was dissolved in 4.5 mL of dimethylformamide (DMF) in a 20 mL vial, which resulted in 33.3% weight/volume. The crosslinker was added to the vial in 1:0.32 (CD:HDI) ratio. The mixture was then poured into a 6 cm Teflon petri dish, covered in Parafilm, and allowed to cure in room temperature for 4 days. An 8 mm diameter punch was used to generate uniform disks out of the polymer, unless otherwise specified. To remove unreacted monomers, the punched polymer disks were placed in a DMF bath for 24 hrs, then a DMF/Water (50/50 % by volume) bath for 24 hrs, and finally a de-ionized water bath for 72 hrs, changing the water every 24 hrs. More details of the protocol can be found in previous literature.29, 30
Sterilization Techniques
Standard sterilization techniques: dry heat, steam (autoclave), gas (ethylene oxide), and radiation (gamma rays), were used to ensure complete irradiation of foreign bodies for effective implantation
Dry heat sterilization was conducted in a sterilization oven that was preheated to 170°C. The samples were placed into the oven, and the temperature equilibrated at 170°C after 10 min. The samples were left in the oven for 30 min to ensure proper heating through the sample. The samples were promptly removed from the oven and stored at room temperature.
Autoclave sterilization was conducted in an in-house autoclave system, which was operated at 121°C for 40 minutes to ensure thorough penetration of the heat and steam throughout the polymer.
For ethylene oxide sterilization, the disks were sent to University Hospital (Cleveland, OH) for a warm ethylene oxide (routine hospital) procedure. Ethylene oxide gas was heated to 38°C at 55% humidity for 12 hrs. Afterwards, the polymers were degassed for 48 hrs to ensure that all the ethylene oxide gas was removed from the polymer.
Gamma irradiated samples were exposed to 25 kGy gamma radiation, (Cobalt 60) via Steris Isomedix standard engineering run.33
After sterilization, all polymers were compared to non-sterilized polymers.
Thermogravimetric Analysis
Thermogravimetric analysis (TGA) was performed on a TA Instruments Q500 TGA with a heating rate of 10°C/min in a nitrogen gas flow of 40 mL/min. Samples of 8±2 mg were used. The weight change and derivative of the weight change was recorded versus time and temperature.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) analysis was performed using a TA instruments Q100 DSC. A sample weight of 3-5 mg was placed in an aluminium pan and sealed hermetically. An aluminium pan was sealed without any sample, using air as a reference. The pans were initially held at −60°C and heated to 250°C under a nitrogen atmosphere. Then they were cooled to 0°C and finally heated again to 250°C.
Tensile-Strength Test
Polymers were punched in the shape of a dumbbell for tensile-strength tests. Dumbell-shaped samples were immersed in phosphate buffer saline (PBS, pH 7.4) for over 24 hrs to ensure complete soaking. Wet conditions were used to more appropriately mimic biological conditions. The samples were blotted dry and placed immediately (less than one minute) into the grips of an Instron Model 1125 Universal Testing apparatus. The samples were pulled at 1 mm/min until complete fracture. The force was measured with a 10 N load cell (resolution 0.01 N). The strain and stress were calculated. Replicas of 4-5 were created to ensure statistical significance.
Drug Loading
Erythromycin, vancomycin, and rifampicin were each loaded into different 8 mm polymer disks after the various sterilization techniques (or in equivalent non-sterilized samples). Erythromycin and rifampicin (5 wt%) were dissolved and loaded in DMF, whereas vancomycin (5 wt%) was dissolved and loaded in water. The disks were placed in the drug solutions and loaded at room temperature for 4 days. The loaded disks were rinsed with sterile water and blotted with a sterile Kimwipe to remove excess solvent and surface drug before being dried through evaporation at room temperature.
Drug Release
All samples were weighed, and then the drug-loaded disks (both previously sterilized and non-sterilized) were placed individually into 20 mL sample vials containing 10 mL of phosphate buffer saline (PBS, pH 7.4). The samples were incubated at 37°C and agitated at 85 rpm in a shaking incubator. Every 24±4 hrs, aliquots (0.5 mL) were withdrawn from each vial and replaced with an equal volume of PBS to ensure constant volume. The concentration of drug in each sample was determined spectrophotometrically at 485 nm (Molecuar Devices Biolumin 960) for rifampicin and 230 nm for vancomycin (Varian 100 Bio UV-VIS Spectrophotometer). Erythromycin required a hot sulphuric acid reaction to turn the chemical a yellowish colour.34 After the reaction, the concentration of drug was read at 480 nm.
Standard curves for the amount of drug in the PBS solution were generated to determine the exact concentration released. Total drug released (100%) was determined as the final total concentration released after an extremely long time with little or no release, and was calculated after the drug release experiments. Each drug release experiment was repeated three times for ANOVA statistical analysis using Excel.
Zone of Inhibition
A zone of inhibition assay, also known as a Kirby-Bauer assay, was performed to test the bioactivity of the antibiotic-loaded sterilized disks versus non-sterilized disks. Agar plates were prepared as previously described.29 A tripticase soy broth culture of log phase Staphylococcus aureus (70 μL) was inoculated evenly over a tripticase soy agar plate using a sterilized spreader. The drug-loaded disks were placed on top of the inoculated plate and then immediately incubated at 37°C. Every 24 hours, the zones of clearance around the disks were recorded, using a calliper, to measure the bioactivity of the disks during that day. The disks were then (daily) transferred to a new bacteria-covered plate.
Results and Discussion
Swelling Ratio Calculations
| (1) |
The swelling ratio was determined by measuring the dry weight and wet weight (after 24 hrs soaking in water), and then the ratio was calculated using equation (1). The swelling ratios of sterilized polymers were found to be significantly different (p<0.0001) from non-sterilized polymers, Table 1. Swelling ratio is typically proportional to drug loading, which in this case indicates that sterilized polymers, especially autoclave (50% less swelling), will have less drug loading. The thermomechanical and drug release changes are further investigated for polymers processed by each sterilization technique.
Table 1.
Swelling ratios of non-sterilized polymers compared to autoclave, ethylene oxide, and gamma radiation sterilized polymers. P-values compared to non-sterilized polymers.
| Sterilization Type | Averag | e St. Dev | P-value |
|---|---|---|---|
| Dry Heat | 0.37 | 0.02 | <0.0001 |
| Autoclave | 0.74 | 0.01 | <0.0001 |
| Ethylene Oxide | 1.30 | 0.02 | <0.0001 |
| Gamma Radiation | 1.34 | 0.01 | <0.0001 |
| Non-Sterilized | 1.52 | 0.02 | 1 |
Thermogravimetric Analysis Results
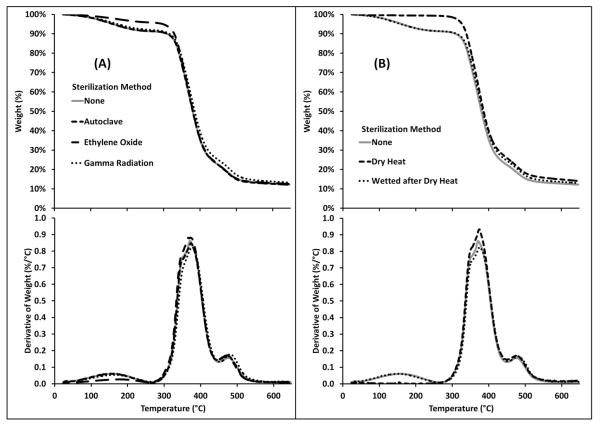
Thermogravimetric analysis (TGA) was conducted on cyclodextrin polymers sterilized by autoclave, ethylene oxide, and gamma radiation and compared to non-sterilized polymers, Figure 1. Figure 1a shows that the various sterilization procedures had minimal effect on the degradation profiles of the polymer. The only measureable difference was observed at the 150°C degradation, which is due to water content. Ethylene oxide is capable of reacting with embedded water content, reducing the observed degradation with TGA at this temperature. The non-sterilized polymers and polymers sterilized by autoclave and gamma radiation had ~10% degradation associated with embedded water content. Non-sterilized and sterilized polymers experienced ~65% degradation associated with cyclodextrin, with the remainder associated with the crosslinker. The degradation profile is consistent with the initial weight of the reactants. Polymers sterilized by dry heat were tested to confirm that the degradation centered at 150°C was associated with water, and that the embedded water content can be recovered, Figure 1b. The polymer was immediately tested after being sterilized by dry heat and no degradation was observed at 150°C. However, after the polymer is exposed to water for 24 hrs and dried, the 150°C peak returns.
Fig 1.
TGA data of cyclodextrin:HDI polymers showing that sterilization has minimal effect on the degradation profiles. Top: weight % vs. temperature. Bottom: Derivative of weight % vs. temperature. (a) Polymers sterilized by autoclave, ethylene oxide, and gamma radiation compared to a non-sterilized polymer. All four plots are identical, except less degradation is observed at 150°C in polymers sterilized by ethylene oxide due to reduced embedded water content. (b) After dry heat sterilization, no degradation exists at 150°C (because embedded water content is evaporated during the dry heat sterilization procedure), but the degradation peak returns after the polymer is wetted and dried overnight.
The TGA results confirm that the embedded water content of all samples is typically 10% unless the embedded water is reacted (e.g. ethylene oxide sterilization) or vaporised (e.g. dry heat sterilization). Also, the degradation profile remains consistent after sterilization, confirming that the sterilization protocols do not degrade or change the general structure of the polymer.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) was conducted on polymers sterilized by autoclave, ethylene oxide, and gamma radiation and compared to non-sterilized polymers, Figure 2a. The cool-down and baseline (second heat-up) curves were found to be uniform with no phase transitions (data not shown). The baseline curve was subtracted from the initial curve, Figure 2, which revealed two non-standard irreversible peaks at 125°C and 235°C. The peak centered at 125°C was identified as embedded water content; the peak centered around 235°C was due to nondescriptive thermal memory. Minimal changes were observed between the sterilized and non-sterilized polymers. The largest difference was found in the amount of embedded water content contained in the ethylene oxide sterilized polymers.
Fig. 2.
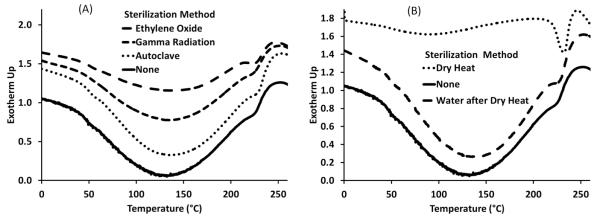
DSC data of cyclodextrin:HDI polymers showing that sterilization has minimal effect on the heating profile and crystallinity.(A) Polymers sterilized by autoclave, ethylene oxide and gamma radiation compared to a non-sterilized polymer. After baseline subtraction, two irreversible peaks, centered at 125°C (embedded water content) and 235°C (non-descriptive thermal memory), are observed. Polymers sterilized with ethylene oxide had less embedded water content; ethylene oxide reacted with the water content inside the polymer. (B) The water peak (125°C) does not exist after polymers are sterilized by dry heat; the water evaporates from the polymer. The peak at 125°C returns once the dried polymer is re-exposed to water.
Polymers sterilized by dry heat were tested directly after exposure to the heat source, Figure 2b; no embedded water content was observed. Dry heat sterilized polymers were then swelled in water and dried at room temperature, and the embedded water content peak returned. The dry heat experiments confirmed that the peak centered at 125°C was due to embedded water content, and the 125°C peak would return after exposure to water.
The DSC results confirmed that the sterilization procedures do not affect the heating profile and crystallinity of the polymers, and no glass transition temperatures were observed.
Tensile-Strength Tests
Tensile-strength tests were conducted on sterilized polymers shaped into dog-bones and compared to the tensile-mechanical strength of non-sterilized polymers, Figure 3. Ethylene oxide and gamma radiation sterilized polymers show no significant difference in mechanical strength compared to non-sterilized polymers. Polymers sterilized by autoclave showed a significant increase in strength (Young’s modulus = 9.4±0.8) as compared to non-sterilized polymers (young’s modulus = 4.3±0.3), Table 2, p<0.0001.
Fig. 3.
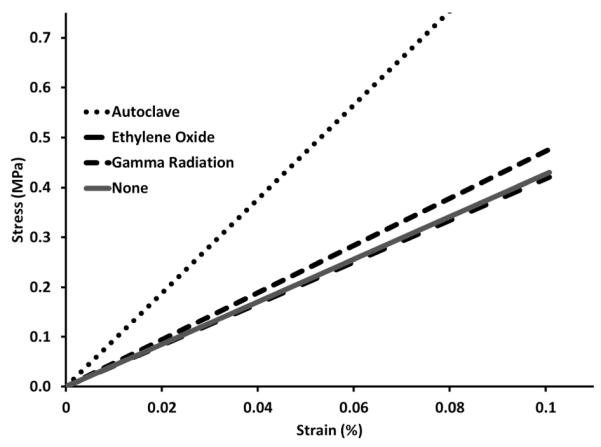
Stress vs. strain plot from tensile tests comparing cyclodextrin:HDI polymers sterilized by gamma radiation, ethylene oxide, and autoclave with non-sterilized polymers to show a change in Young’s modulus (tensile strength). A significant increase in tensile strength is observed in polymers sterilized by autoclave (N=5, p<0.00001, Table2).
Table 2.
Young’s modulus of cyclodextrin-HDI polymers after exposure to sterilization. The p-values are compared to non-sterilized polymers.
| Sterilization Technique |
Young’s Modulus (MPa) |
St. Deviation | P-value |
|---|---|---|---|
| Autoclaved | 9.4 | 0.8 | <0.00001 |
| Ethylene Oxide | 4.2 | 0.3 | 0.6 |
| Gamma Radiation | 4.7 | 0.3 | 0.05 |
| Non-Sterilized | 4.3 | 0.3 | 1 |
The increase in tensile strength and decrease in swelling ratio of polymers sterilized by autoclave, Table 1, is indicative of greater crosslinking as compared to non-sterilized polymers. Although a decrease in the swelling ratios for polymers sterilized by ethylene oxide and gamma radiation are observed, there seems to be minimal change in the tensile strength. The density of crosslinking may be affected from ethylene oxide and gamma radiation sterilization, but not to a degree that affects the tensile-mechanical strength.
Drug Release Profiles
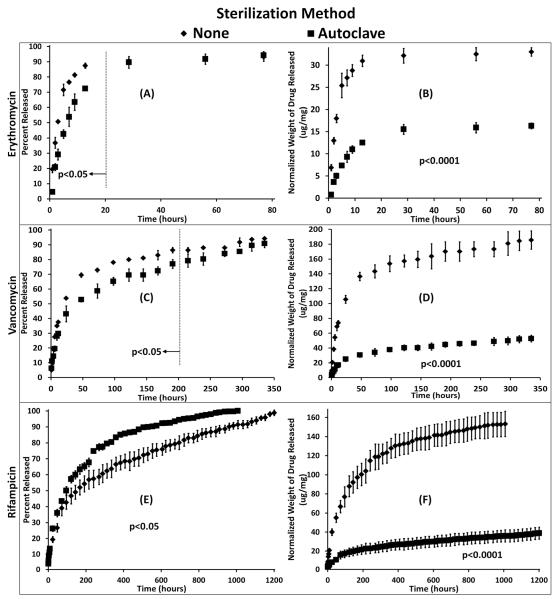
The release of drugs as a function of time from polymers sterilized by autoclave compared to non-sterilized control polymers can be seen in Figure 4. Differences in the release profiles between erythromycin (Figure 4 A,B), vancomycin (Figure 4 C,D), and rifampicin (Figure 4 E,F) are due to differences between the hydrophobicity of the drug which impacts affinity, and therefore, how well the drug binds with cyclodextrin. Polymers sterilized by autoclave released a significantly lower initial percentage of drug compared to the controls (p<0.05). Also, polymers sterilized by autoclave continued to release drug after the non-sterilized polymers reached 100% release. Thus, the polymers sterilized by autoclave showed a longer, slower release profile than the non-sterilized polymers. While the slower release of polymers sterilized by autoclave was present in the release profile of each antibiotic, it was most evident in the vancomycin and rifampicin release curves.
Fig. 4.
Drug released from cyclodextrin:HDI polymers sterilized by autoclave compared to non-sterilized that were loaded with (A, B) erythromycin, (C, D) vancomycin, and (E, F) rifampicin after sterilization. (Left) The percentage of drug released slowed significantly after autoclaving (p<0.05), and (Right) the total amount of drug released was significantly lower in autoclaved polymers compared to non-sterilized controls (p<0.0001). Each drug releases at a different rate and therefore is presented on a different time scale.
Polymers sterilized by autoclave also displayed a significant decrease in the total amount of drug released compared to the non-sterilized controls (p<0.0001) due to a decrease in drug loading (Table 1). Erythromycin exhibited approximately a 2-fold decrease, and vancomycin and rifampicin exhibited approximately a 4.5 fold decrease comparing non-sterilized polymers with polymers sterilized by autoclave.
Based on the tensile-stress tests and the release data, the autoclave sterilization protocol caused further reaction of the cross-linker, creating a denser cyclodextrin network. A higher density of crosslinking would cause less polymer swelling (Table 1) and less drug to be loaded into the autoclaved disks (Figure 4 B, D, F), as indicated in previous literature.30 The higher crosslinking density would result in a higher diffusion coefficient (i.e. reduced release rate), Figure 4 A, C, E) creating a longer release profile..
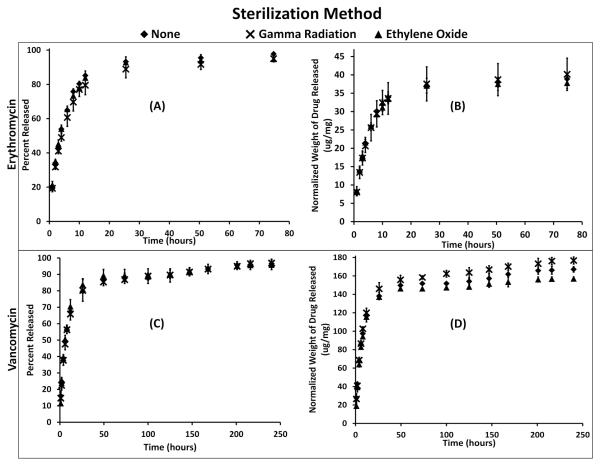
The release of drug versus time was also evaluated for polymers sterilized by ethylene oxide and gamma radiation, Figure 5. Polymer disks sterilized by ethylene oxide and gamma radiation showed no significant change from the release profile compared to non-sterilized polymers for each antibiotic, Figure 5 A, C. No significant change, between polymers sterilized with ethylene oxide or gamma radiation and non-sterilized polymers, in the total amount of drug released, Figure 5 B, D. Based on the release profiles and the tensile-strength test results (Figure 3 and Table 2), the gamma radiation and ethylene oxide sterilization protocols had no significant effect on the properties of the cyclodextrin-HDI crosslinked drug delivery process.
Fig. 5.
Drug released from cyclodextrin:HDI polymers sterilized by ethylene oxide and gamma radiation compared to non-sterilized that were loaded with (A, B) erythromycin and (C, D) vancomycin after sterilization. No statistical difference was found in the (Left) rate of drug released or (Right) the quantity of drug released from the gamma radiation sterilized polymers, ethylene oxide sterilized polymers, and non-sterilized controls. Each drug releases at a different rate and therefore is presented on a different time scale.
Zone of Inhibition
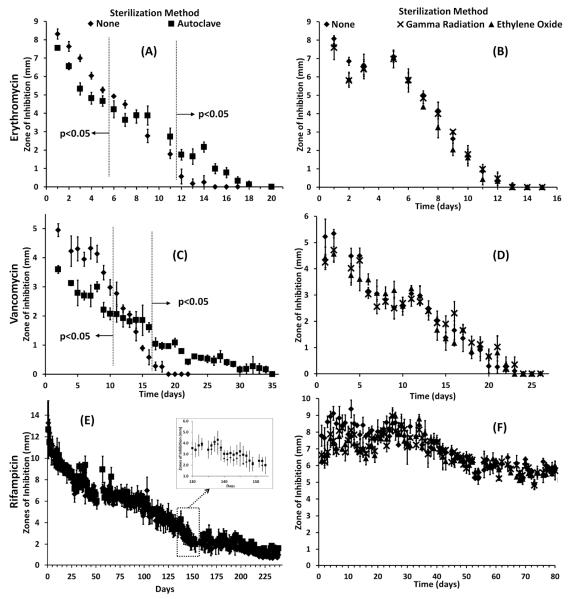
The bactericidal activity of sterilized and non-sterilized polymers was measured in terms of the radius of the zone of inhibition, Figure 6. Polymers sterilized by autoclave, that were loaded with erythromycin and vancomycin, initially showed less bactericidal activity than the non-sterilized disks; both had an initial smaller zone of inhibition before Day 6 (erythromycin) and Day 10 (vancomycin), Figure 6 A, C. The initial lower activity of polymers sterilized by autoclave is probably due to less swelling and less drug loading compared to non-sterilized polymers. Midway through the release of the antibiotics, polymers sterilized by autoclave, polymers had similar zone sizes compared to non-sterilized polymers. The bactericidal activity of drug released from polymers sterilized by autoclave was significantly larger after 11 days (erythromycin) and 16 days (vancomycin) for erythromycin and vancomycin release. In addition, zone sizes of non-sterilized disks reached zero before the polymers sterilized by autoclave; polymers sterilized by autoclave continued to show bactericidal activity after the non-sterilized polymers had ceased showing bactericidal activity. Even though less of the drug was initially available, zone of inhibition experiments confirmed that the longer release of erythromycin and vancomycin antibiotics for polymers sterilized by autoclave still had drug sufficient to clear bacteria.
Fig. 6.
Zone of inhibition (Kirby-Bauer) assay from cyclodextrin:HDI polymers sterilized by autoclave, ethylene oxide, and gamma radiation compared to non-sterilized polymers that were loaded with (A, B) erythromycin, (C, D) vancomycin, and (E, F) rifampicin after sterilization. (A) The bactericidal activity of autoclaved gels with erythromycin is significantly smaller for the first five days (p<0.05) yet significantly larger after 11 days (p<0.05). (C) The bactericidal activity of autoclaved gels with vancomycin is significantly smaller for the first 10 days (p<0.05) yet significantly larger after 16 days (p<0.05). (E) Bactericidal activity of rifampicin loaded autoclave sterilized disks was similar to that of non-sterilized disks for over 8 months (243 days). (B, D, F)Bactericidal activity from ethylene oxide or gamma radiation sterilized, drug-loaded polymers showed no difference from the activity from non-sterilized polymers loaded with erythromycin, vancomycin, or rifampicin.
As expected, based on the release rate curves, the bactericidal activity of erythromycin and vancomycin from polymers sterilized by ethylene oxide and gamma radiation was no different than non-sterilized polymers, Figure 6 B, D. The unchanged profile indicates that the polymers sterilized by ethylene oxide and gamma radiation will perform as evaluated in non-sterilized samples.
Rifampicin release from polymers sterilized by autoclave was not significantly different from non-sterilized polymers over 8 months (243 days), Figure 6E. During the rifampicin release, affinity interactions dominate over diffusion because the drug binds strongly with the cyclodextrin-based polymer;30 therefore, the increase in the diffusivity coefficient (expected due to minor changes in crosslinking) does not significantly affect the long-term affinity-based bactericidal activity.
Polymers sterilized by ethylene oxide and gamma radiation that were loaded with rifampicin also had similar bactericidal activity compared to non-sterilized polymers, Figure 6F, up to 80 days.
Conclusions
Release profiles and bactericidal activity of sterilized cyclodextrin polymers were compared to non-sterilized polymers. Three drugs were tested, erythromycin, vancomycin, and rifampicin, to give a representation of how drugs with different binding to cyclodextrin interacts with the sterilized polymer. Further, these drugs indicate how future drugs will interact with a cyclodextrin polyurethanes.
Although polymers sterilized by ethylene oxide and gamma radiation showed a minor increase in polymer swelling after sterilization, no significant change was observed in the tensile-strength, release rate, or zone of inhibition of the polymer.
Polymers sterilized by autoclave showed a significant reduction in the total amount of drug loaded and released, presumed to be due to the increase in crosslinking density (as determined previously); however, this only affected the initial release rate of the antibiotic. Of the drugs tested, only the ones with a lower affinity to cyclodextrin (vancomycin and erythromycin) showed any difference between autoclaved polymers and non-sterilized polymers in terms of zone of inhibition. The antibiotic with the highest affinity (rifampicin) showed virtually no difference in zone of inhibition between autoclaved and non-sterilized polymers for over 8 months.
The tests on sterilized polymers revealed two important results. First, ethylene oxide and gamma radiation sterilization techniques can be used without significantly modifying the polymer properties. Second, autoclave sterilization can be used to increase crosslinking density, thereby allowing superior control over the release properties and bactericidal activity of loaded polyurethanes. Even with a reduced total loading amount, autoclave sterilization can be used when a prolonged release is desired over greater initial release or drug loading.
Acknowledgments
This investigation was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 AR007505 from the NIH NIAMS, the Innovation Incentive Program administered by the Advanced Platform Technology Center within Veteran Affairs Hospitals, and von Recum’s REU supplement of his NSF grant (CBET-1235738).
Notes and References
- 1.Athanasiou KA, Niederauer GG, Agrawal CM. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 2.Razem D, Katusin-Razem B. Radiat. Phys. Chem. 2008;77:288–344. [Google Scholar]
- 3.Grabow N, Schlun M, Sternberg K, Hakansson N, Kramer S, Schmitz K-P. Journal of Biomechanical Engineering. 2005;127:25–31. doi: 10.1115/1.1835349. [DOI] [PubMed] [Google Scholar]
- 4.Puppi D, Chiellini F, Piras AM, Chiellini E. Progress in Polymer Science. 2010;35:403–440. [Google Scholar]
- 5.Hoffman AS. Advanced Drug Delivery Reviews. 2012;64:18–23. [Google Scholar]
- 6.Middleton J, Tipton AJ. Biomaterials. 2000;21:2335–2346. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 7.Calis S, Bozdag S, Kas HS, Tuncay M, Hincal AA. Il Farmaco. 2002;57:55–62. doi: 10.1016/s0014-827x(01)01171-5. [DOI] [PubMed] [Google Scholar]
- 8.Friess W. European Journal of Pharmaceutics and Biopharmaceutics. 1998;45:113–136. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 9.Gibas I, Janik H. Chemistry & Chemical Technology. 2010;4:297–304. [Google Scholar]
- 10.Odelius K, Plikk P, Albertsson A-C. Biomaterials. 2008;29:129–140. doi: 10.1016/j.biomaterials.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 11.Gogolewski S, Mainil-Varlet P. Biomaterials. 1996;17:523–528. doi: 10.1016/0142-9612(96)82727-x. [DOI] [PubMed] [Google Scholar]
- 12.Gogolewski S, Mainil-Varlet P. Biomaterials. 1997;18:251–255. doi: 10.1016/s0142-9612(96)00132-9. [DOI] [PubMed] [Google Scholar]
- 13.Fouad H, Elsarnagawy T, Almajhdi FN, Khalil KA. Int. J. Electrochem. Sci. 2013;8:2293–2304. [Google Scholar]
- 14.Reich MS, Akkus O. Cell Tissue Bank. 2012 doi: 10.1007/s10561-012-9335-z. [DOI] [PubMed] [Google Scholar]
- 15.Koo G-H, Jang J. Journal of Applied Polymer Science. 2013:4515–4523. [Google Scholar]
- 16.Lerouge S, Wertheimer MR, Yahia LH. Plasmas and Polymers. 2001;6:175–188. [Google Scholar]
- 17.Darmady EM, Hughes KEA, Jones JD, Prince D, Tuke W. J. Clin. Path. 1961;14:38–44. doi: 10.1136/jcp.14.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruel-Gariepy E, Leroux J-C. European Journal of Pharmaceutics and Biopharmaceutics. 2004;58:409–426. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Zahraout C, Sharrock P. Bone. 1999;25:63S–65S. doi: 10.1016/s8756-3282(99)00136-2. [DOI] [PubMed] [Google Scholar]
- 20.Phillip E, Jr., Murthy NS, Bolikal D, Narayanan P, Kohn J, Lavelle L, Bodnar S, Pricer K. Journal of Biomed Mater Res Part B. 2013 doi: 10.1002/jbm.b.32853. [DOI] [PubMed] [Google Scholar]
- 21.Mendes GCC, Brandao TRS, Silva CLM. Am. J. Infect. Control. 2007;35:574–581. doi: 10.1016/j.ajic.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Ishigaki I, Yoshii F. Radiat. Phys. Chem. 1992;39:527–533. [Google Scholar]
- 23.Chmielewski AG, Haji-Saeid M, Ahmed S. Nuclear Instraments and Methods in Physics Research B. 2005;236:44–54. [Google Scholar]
- 24.Goldman M, Gronsky R, Ranganathan R, Pruitt L. Polymer. 1996;37:2909–2913. [Google Scholar]
- 25.Juan AS, Montembault A, Gillet D, Say JP, Rouif S, Bouet T, Royaud I, David L. Materials Science and Engineering. 2012;31:1–5. [Google Scholar]
- 26.Volland C, Wolff M, Kissel T. Journal of Controlled Release. 1994;31:293–305. [Google Scholar]
- 27.Thatiparti TR, Averell N, Overstreet D, von Recum HA. Macromol. Biosci. 2011;11:1544–1552. [PubMed] [Google Scholar]
- 28.Harth KC, Rosen MJ, Thatiparti TR, Jacobs MR, Halaweish I, Bajaksouzian S, Furlan J, von Recum HA. Association for Academic Surgery. 2010;163:337–343. doi: 10.1016/j.jss.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 29.Thatiparti TR, Shoffstall AJ, von Recum HA. Biomaterials. 2010;31:2335–2347. doi: 10.1016/j.biomaterials.2009.11.087. [DOI] [PubMed] [Google Scholar]
- 30.Thatiparti TR, von Recum HA. Macromol. Biosci. 2010;10:82–90. doi: 10.1002/mabi.200900204. [DOI] [PubMed] [Google Scholar]
- 31.Merritt SR, Velasquez G, von Recum HA. Experimental Eye Research. 2013;116:9–16. doi: 10.1016/j.exer.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirata N, Mattsumoto K.-i., Inishita T, Takenaka Y, Suma Y, Shintani H. Radiat. Phys. Chem. 1995;46:377–381. [Google Scholar]
- 33.Nguyen H, Morgan DAF, Forwood MR. Cell and Tissue Banking. 2007;8:81–91. doi: 10.1007/s10561-006-9019-7. [DOI] [PubMed] [Google Scholar]
- 34.Ford JH, Prescott GC, Hinman JW, Caron EL. Analytical Chemistry. 1953;25:1195–1197. [Google Scholar]