Abstract
Purpose:
To evaluate the negative predictive value (NPV) of ultrasound (US) elastography for non-palpable Breast Imaging Reporting and Data System (BI-RADS) category 3 lesions on ultrasonography and to determine whether US elastography is helpful in reducing the number of BI-RADS category 3 lesions on ultrasonography.
Methods:
Two hundred seventy-six consecutive, non-palpable BI-RADS category 3 lesions in 256 women who underwent US elastography and US-guided core biopsy, and who had at least 12 months of follow-up data, comprised our study group. The BI-RADS final assessment category and elasticity score were prospectively and independently classified. The rate of malignancy and NPV according to the elasticity score were analysed. We also investigated whether there was a subset of BI-RADS category 3 lesions that were of benign histology but negative on elastography.
Results:
Of the 276 non-palpable BI-RADS category 3 lesions, three lesions (1.0%) were finally confirmed as ductal carcinomas in situ. No cancers were found in the remaining 273 lesions with benign biopsy histology at a mean follow-up of 39.4 months (range, 12 to 72 months). The NPV of a negative elasticity score (elasticity score of 1) was 99.3% (165 of 166). If BI-RADS category 3 lesions showing a negative elasticity score were downgraded to BI-RADS category 2, 60.4% (165 of 273) of them with benign histology could have been safely followed without biopsy with an increased malignancy rate from 1% (3 of 276) to 1.8% (2 of 110), which is not significantly higher (P=0.626).
Conclusion:
US elastography has the potential to reduce the number of BI-RADS category 3 lesions on ultrasonography.
Keywords: Breast neoplasms, Ultrasonography, Elasticity imaging techniques, Biopsy
Introduction
With the expanded role of screening breast ultrasonography and accumulating clinical experience, the American College of Radiology (ACR) has established the Breast Imaging Reporting and Data System(BI-RADS) ultrasonography lexicon to standardize terminology for description and management recommendations according to stratified risks [1]. In the field of mammography screening, it has been established that probably benign (BI-RADS category 3) lesions having a malignancy rate of less than 2% require shortterm imaging follow-up rather than immediate biopsy [2,3]. Although BI-RADS category 3 criteria have been validated for noncalcified, circumscribed oval masses on both mammography and ultrasonography [3,4], there has been no validation for such lesions found on ultrasonography. Their incidences on ultrasonography have been reported to range from 15.7% to 32.2% [4-7], and their malignancy rate from 0% to 2.6% [4-10]. A recent prospective study suggested an annual follow-up recommendation rather than short-term follow-up for non-palpable BI-RADS category 3 lesions on ultrasonography based on their low malignancy rate, low incidence of suspicious changes at 6-month follow-up, and early stage of detected cancers [8].
Another approach to reduce unnecessary short-term follow-ups or biopsies and to increase the specificity of supplemental screening ultrasonography is the use of ultrasound (US) elastography. US elastography has been reported to be useful to distinguish benign from malignant lesions [11-18]. Recent studies also have suggested that US elastography might be helpful for further characterizing lesions with low suspicion and thereby reducing the need for biopsies with benign results [6,14-18]. However, to the best of our knowledge, there have been few studies regarding the application of US elastography in reducing the number of BI-RADS category 3 lesions [19]. Thus, the purpose of this study were to evaluate the negative predictive value (NPV) of US elastography for non-palpable BI-RADS category 3 lesions on ultrasonography and to determine whether US elastography is helpful in reducing the number of BIRADS category 3 lesions on ultrasonography.
Materials and Methods
Patients and Breast Masses
Our institutional review board approved this study, and informed consent was obtained from each woman before the biopsy. From May 2006 through June 2009, 2,107 consecutive women underwent B-mode ultrasonography, US elastography, and a subsequent US-guided needle biopsy of 2,527 non-palpable breast masses. The inclusion criteria were as follows: (1) non-palpable breast lesions classified as BI-RADS category 3 based on B-mode ultrasonography, (2) lesions that have undergone US elastography, (3) lesions that have undergone US-guided core biopsy, (4) lesions that were followed for at least 1 year after US-guided biopsy. Among the 2,527 lesions, 299 lesions were classified as BI-RADS category 3 on B-mode breast ultrasonography in 273 women. Sixteen lesions were excluded owing to unavailability of 1-year follow-up data. Seven lesions were additionally excluded due to a lesion size larger than 30 mm, as sufficient normal tissue around the target lesion was needed to obtain adequate strain images [20]. Therefore, the size criteria for final inclusion was a lesion of 30 mm or less. A total of 276 breast masses (mean size, 9.8 mm; range, 3 to 26 mm) in 256 women (mean age, 45.6 years; range, 24 to 67 years) were included in the final data analysis. Of the 256 women, 231 women (90.2%) had no symptoms, 23 women (8.9%) had pain, and two women (0.7%) had bilateral milky nipple discharge. These women underwent biopsies even without any clinically suspicious features due to the request of the woman or the referring clinician.
B-mode Ultrasonography
B-mode ultrasonography was performed using a 14- to 6-MHz linear transducer of a EUB-8500 (Hitachi Medical, Tokyo, Japan) ultrasonography system. One of nine radiologists with 1-10 years’ experience in performing and interpreting breast ultrasonography and mammography performed the ultrasonography after reviewing available mammographic images. Of the 256 women, 251 (98.0%) had available mammograms at the time of ultrasonography. All mammographic findings were correlated with US findings during the ultrasonography. Of the 251 mammograms, 1 (0.3%) had an almost entirely fatty breast (BI-RADS 1 density), 47 (18.7%) had scattered fibroglandular tissue in fatty breasts (BI-RADS 2 density), 176 (70.1%) had heterogeneously dense breasts (BI-RADS 3 density), and 27 (10.7%) had extremely dense breasts (BI-RADS 4 density). Of the 251 mammograms, no abnormality was found in 167 (66.5%), an asymmetry or a mass was found in 47 (18.7%), and microcalcifications with or without a mass or asymmetry were found in 37 (14.7%). After a mass was identified on B-mode ultrasonography, radial and antiradial scans were performed for accurate characterization. The final assessment category of B-mode ultrasonography was prospectively determined, and less than 2% of the cases were classified in BI-RADS category 3 with expected malignancy risk. Regarding the criteria for BI-RADS category 3, a solid mass without any suspicious features and showing an oval shape, circumscribed margin, parallel orientation, and no posterior features or minimal enhancement was considered to be probably benign [4]. Clustered microcysts, complicated cysts, or postop scars were also considered probably benign [1]. It was recommended that those lesions be followed up at 6, 12, and 24 months in our clinical practice. However, when referring physicians or patients requested a biopsy for the lesions, US-guided core biopsy was performed.
US Elastography
One of nine radiologists performed US elastography at the time of the scheduled biopsy procedure. The mean time interval between the previous B-mode ultrasonography and US elastography was 26 days (range, 0 to 356 days). There were three cases with an interval between the previous B-mode ultrasonography and US elastography of more than 180 days, but none of those cases showed a lesion size or category change. All of the radiologists had experience with more than 100 cases of US elastography, which was obtained using a 14- to 16-MHz linear transducer with a EUB-8500 machine, as performed in a previous study [13]. A five-point elasticity score suggested by a previous study [11] was prospectively classified and recorded in the medical records of US-guided core biopsy on a picture archiving and communication system as follows. A score of 1 indicated uniform strain for the entire hypoechoic lesion (i.e., the image of the entire lesion was evenly shaded in green). A score of 2 indicated strain in most of the hypoechoic lesion with some areas of no strain (i.e., the hypoechoic lesion had a mosaic pattern of green and blue). A score of 3 indicated strain at the periphery of the hypoechoic lesion with sparing of the centre of the lesion (i.e., the peripheral part of the lesion was green, and the central part was blue). A score of 4 indicated no strain in the entire hypoechoic lesion (i.e., the entire hypoechoic lesion was blue, but its surrounding area was green). A score of 5 indicated no strain in the entire hypoechoic lesion or in the surrounding area (i.e., both the entire hypoechoic lesion and its surrounding area were blue). Immediately after the US elastographic examinations, the radiologist performed USguided needle biopsy using a 14-gauge automated gun (Pro-Mag 2.2, Manan Medical Products, Northbrook, IL, USA) or an 11-gauge vacuum-assisted device.
Data Analysis
A blinded radiologist who had not performed the conventional ultrasound or US elastography analysed the data as follows: first, elasticity scores according to the histologic results of 276 lesions were tabulated, and the mean elasticity scores according to the histological types were calculated. Second, the rate of malignancy, sensitivity, specificity, positive predictive value (PPV), and NPV according to the elasticity score were analysed. Sensitivity and specificity were evaluated with the cut-off value between the elasticity scores of 1 and 2 as performed previously to prevent a decrease in the sensitivity of US elastography [14]. Lastly, we investigated whether a subset of the BI-RADS category 3 lesions on B-mode ultrasonography could be downgraded to category 2 upon the addition of US elastography. The statistical analyses were performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Differences with a P-value of less than 0.05 were considered significant.
Results
Elasticity Scores According to Histopathology
Of the 276 lesions, three lesions (1.0%) were malignant and 273 (98.9%) were benign, which was confirmed by 14-gauge core needle biopsy (n=224), 11-gauge vacuum-assisted biopsy (n=42), or US-guided surgical excision (n=7). All 273 benign lesions were followed for a mean of 39.4 months (range, 12 to 72 months), and their lesion stability was confirmed. Fifty-six of the 273 benign lesions (20.5%) were followed for 12-23 months. The malignant lesions comprised three ductal carcinomas in situ (DCISs). Two of them were initially reported to be atypical ductal hyperplasia (ADH) at core biopsy and were confirmed as DCIS by subsequent surgical excision. Regarding the benign papillomas, there were 5 intraductal papillomas, and all of the papillomas were confirmed to be benign by surgical excision (n=3) or by their stability for at least 3 years of follow-up (n=2). The mean lesion size, defined as the maximal diameter measured on ultrasonography, of the malignant masses was 14.3±9.7 mm (range, 6 to 25 mm), and the mean size of the benign masses was 9.8±4.9 mm (range, 3 to 26 mm).
The most common histologic findings of 276 lesions with BI-RADS category 3 were a fibrocystic change (111, 40.2%) followed by a fibroadenoma (87, 31.5%) (Table 1). An elasticity score of 1 (60.1%, 165 of 276) was the most common score for benign lesions (Fig. 1), whereas each of the three malignant masses showed elasticity scores of 1, 2, and 5 (Table 2).
Table 1.
Histopatholoy of BI-RADS category 3 lesions according to the elasticity score
| Histology | Elasticity score |
||||||
|---|---|---|---|---|---|---|---|
| Mean±SD | 1 | 2 | 3 | 4 | 5 | Total | |
| Fibrocystic change | 1.37±0.56 | 74 (26.8) | 33 (11.9) | 4 (1.4) | 0 | 0 | 111 (40.2) |
| Fibroadenoma | 1.55±0.66 | 47 (17.0) | 32 (11.5) | 8 (2.8) | 0 | 0 | 87 (31.5) |
| Intraductal papilloma | 1.60±0.89 | 3 (1.0) | 1 (0.3) | 1 (0.3) | 0 | 0 | 5 (1.8) |
| Usual ductal epithelial hyperplasia | 1.67±0.62 | 6 (2.1) | 8 (2.8) | 1 (0.3) | 0 | 0 | 15 (5.4) |
| Adenosis | 1.50±0.67 | 7 (2.5) | 4 (1.4) | 1 (0.3) | 0 | 0 | 12 (4.3) |
| Granuloma | 1.0 | 1 (0.3) | 0 | 0 | 0 | 0 | 1 (0.3) |
| Fibrosis | 1.50±0.71 | 1 (0.3) | 1 (0.3) | 0 | 0 | 0 | 2 (0.7) |
| Fat necrosis | 1.67±0.58 | 1 (0.3) | 2 (0.7) | 0 | 0 | 0 | 3 (1.0) |
| Nonspecific benign diseasea) | 1.39±0.60 | 25 (9.0) | 10 (3.6) | 2 (0.7) | 0 | 0 | 37 (13.4) |
| Cancer | 2.67±2.08 | 1 (0.3) | 1 (0.3) | 0 | 0 | 1 (0.3) | 3 (1.0) |
| Total | 166 (60.1) | 92 (33.3) | 17 (6.1) | 0 | 1 (0.3) | 276 (100) | |
Values are presented as number (%) of all 276 lesions.
BI-RADS, Breast Imaging Reporting and Data System.
Nonspecific benign disease includes adipose tissue, ductal dilation, or benign mammopathy.
Figure 1. A 50-year-old woman with a Breast Imaging Reporting and Data System (BI-RADS) category 3 lesion on supplemental screening ultrasonography.
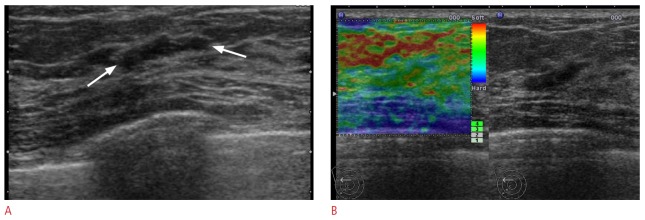
A. Transverse B-mode ultrasonogram shows an 11-mm, oval, circumscribed mass (arrows). B. Ultrasound (US) elastographic image shows the entire mass as green, indicating an elasticity score of 1. US-guided core biopsy revealed a fibrocystic change. Thirty-six month follow-up ultrasonography showed the lesion was not changed (not shown).
Table 2.
Characteristics of the three malignant BI-RADS category 3 lesions
| No. | Age (yr) | Size (mm) | E-score | Histology at core needle biopsy | Histology at surgery | Mammographic findings |
|---|---|---|---|---|---|---|
| 1 | 48 | 25 | 1 | Atypical ductal hyperplasia | 4.5 cm, DCIS, cribriform and papillary type, low grade | Asymmetry |
| 2 | 39 | 12 | 5 | Atypical ductal hyperplasia involving papilloma | 1.5 cm, DCIS, papillary type, low grade | Negative |
| 3 | 49 | 6 | 2 | DCIS, low grade | 1.0 cm, mixed DCIS and LCIS | Negative |
BI-RADS, Breast Imaging Reporting and Data System; E-score, elasticity score; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
Rate of Malignancy According to the Elasticity Score
The overall malignancy rate of BI-RADS category 3 lesions was 1.0% (3 of 276). The rate of malignancy according to the elasticity score was 0.6% (1 of 166) for a score of 1, 1.0% (1 of 92) for a score of 2, 0% (0 of 17) for a score of 3, and 100% (1 of 1) for a score of 5. No lesion showed an elasticity score of 4. When a cut-off value was defined as between an elasticity score of 1 vs. 2, 3, 4, or 5, and the elasticity score of 1 was defined as negative and the elasticity score of 2, 3, 4, or 5 as positive, US elastography showed a sensitivity of 66.6% (2 of 3), specificity of 60.4% (165 of 273), PPV of 1.8% (2 of 110), and NPV of 99.3% (165 of 166).
Downgrading from BI-RADS Category 3 to Category 2 Based on US Elastography
There were three cancer cases with 276 BI-RADS category 3 lesions. One of each showed an elasticity score of 1, 2, and 5 (Tables 1, 2; Figs. 2, 3). Thus, if BI-RADS category 3 lesions showing an elasticity score of 1 were downgraded to BI-RADS category 2, 60.4% (165 of 273) of BI-RADS category 3 lesions with benign histology could have been downgraded, without a significantly increased malignancy rate, that is, from 1% (3 of 276) to 1.8% (2 of 110; P=0.626).
Figure 2. A 48-year-old woman with a Breast Imaging Reporting and Data System (BI-RADS) category 3 lesion on supplemental screening ultrasonography.
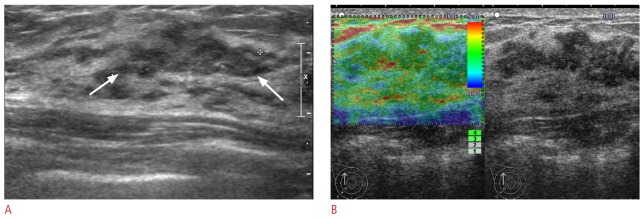
A. Longitudinal B-mode ultrasonogram shows a 25-mm, isoechoic, microlobulated mass (arrows). B. Ultrasound (US) elastographic image shows the entire lesion evenly shaded in green, indicating an elasticity score of 1. A US-guided core biopsy revealed an atypical ductal hyperplasia, which was confirmed to be a low grade ductal carcinoma in situ by subsequent excision (case 1 on Table 2).
Figure 3. A 39-year-old woman with a Breast Imaging Reporting and Data System (BI-RADS) category 3 lesion on supplemental screening ultrasonography.
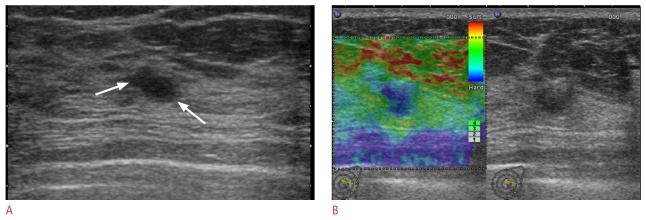
A. Transverse B-mode ultrasonogram shows a 12-mm, oval, indistinct mass (arrows). B. Ultrasound (US) elastographic image shows the entire mass shaded in blue, indicating an elasticity score of 5. A US-guided core biopsy revealed an atypical ductal hyperplasia involving papilloma, which was confirmed to be a low grade ductal carcinoma in situ by subsequent excision (case 2 on Table 2).
Discussion
According to our study, US elastography has the potential in reducing the number of BI-RADS category 3 lesions on ultrasonography. When lesions with BI-RADS category 3 showing a negative elasticity score were downgraded to category 2, 60.4% (165 of 273) of the BI-RADS category 3 lesions with benign histology could have been reduced without a significantly increased malignancy rate from 1% (3 of 276) to 1.8% (2 of 110; P=0.626), leading to substantial cost savings and reduced morbidity.
Our results are similar to those of a recent study using shear- wave elastography in the characterization of BI-RADS category 3 lesions [19]. They found that the criterion of an E-color of ‘black to dark blue’ allowed for the downgrading of 36.3% (110 of 303) of the benign masses without a significant increase in the malignancy rate, that is, from 2.6% to 4.1% [19]. However, controversy remains whether this application is recommendable, given the increased malignancy rate from 2.6% to 4.1%, which is more than the recommended rate for BI-RADS category 3. However, in our study, the rate of malignancy of 1.8% is within the recommended rate. In addition, all three cancer lesions in our population were low grade DCIS, which might be found to still carry a favourable prognosis that is equivalent to that of cancers detected by screening.
One of the main barriers to the implementation of supplemental screening ultrasonography is its high false positive findings of BIRADS category 3 or 4 lesions, leading to benign biopsy results [21]. Recent prospective studies regarding screening ultrasonography reported that approximately 20% (187 of 935, 519 of 2,662) of patients had BI-RADS category 3 lesions in the Connecticut study and also in the ACRIN 6666 study [7,8]. Their rates of malignancy were very low, 0% and 0.8% (6 tumors), respectively. Furthermore, among detected lesions in the ACRIN 6666 study, only one had suspicious changes at the 6-month follow-up, and another one showed a suspicious change at the 1-year follow-up, both of which were node-negative invasive cancers [8]. Based on this observation, they suggested that a recommendation of yearly follow-up for BI-RADS category 3 lesions might be appropriate. Similar to these results, our study also showed a 1% malignancy rate and the detected cancers were all low grade DCIS. Considering that radiologists are opting to classify a substantial number of solid masses appearing benign on screening ultrasonography as BIRADS category 4 and to perform core biopsy, as the radiologists want to avoid the anxiety of missing cases in asymptomatic women, scrutinizing the criteria for downgrading from BI-RADS category 3 to category 2 on B-mode ultrasonography would be beneficial to reduce the number of false positives.
All three malignant lesions out of BI-RADS category 3 were low grade DCIS in our study. This result can be explained by the fact that DCIS tends to show less suspicious features than invasive cancers on B-mode ultrasonography [22], tending to be classified as BI-RADS category 3. Furthermore, as DCIS is softer than invasive cancers [16], it is more difficult to distinguish DCIS from benign lesions than to distinguish invasive cancers from benign lesions on US elastography. In a previous study using the same elasticity score as our study, although the mean elasticity score of DCIS was higher than that of benign disease (2.54±0.11 vs. 1.78±0.81; P<0.001), the mean elasticity score of DCIS was lower than that of invasive ductal carcinomas (2.54±0.11 vs. 3.26±0.09; P<0.001) [14]. Therefore, to minimize false negative cases by downgrading from BI-RADS 3 to BI-RADS 2 based on US elastography, an improved algorithm for US elastography to stratify subtle differences between DCIS and benign lesions is needed.
The present study has several limitations. First, there may have been verification bias, as 20.5% (56 of 273) of the benign lesions were followed for less than 24 months. Second, there may have been selection bias, as we included BI-RADS category 3 lesions, which were scheduled for US-guided core biopsy. Therefore, an unidentified family history or other risk factors motivating patients opting to undergo biopsy might have increased the rate of malignancy in our study group. In addition, the generalizability of our results to the entire spectrum of BI-RADS category 3 lesions encountered in daily practice is limited due to this selection bias. Third, this was a non-randomized study from a single institution. In addition, US elastography and B-mode ultrasonography images could not be evaluated in a completely independent manner, which might have affected the categorization of each finding.
In conclusion, US elastography has the potential to reduce the number of BI-RADS category 3 lesions on ultrasonography by allowing them to be downgraded from BI-RADS category 3 to category 2 when they show a negative elasticity score.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported
Reference
- 1.American College of Radiology . Breast Imaging Reporting and Data System, BI-RADS: Ultrasound. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 2.Varas X, Leborgne JH, Leborgne F, Mezzera J, Jaumandreu S, Leborgne F. Revisiting the mammographic follow-up of BI-RADS category 3 lesions. AJR Am J Roentgenol. 2002;179:691–695. doi: 10.2214/ajr.179.3.1790691. [DOI] [PubMed] [Google Scholar]
- 3.Sickles EA. Nonpalpable, circumscribed, noncalcified solid breast masses: likelihood of malignancy based on lesion size and age of patient. Radiology. 1994;192:439–442. doi: 10.1148/radiology.192.2.8029411. [DOI] [PubMed] [Google Scholar]
- 4.Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123–134. doi: 10.1148/radiology.196.1.7784555. [DOI] [PubMed] [Google Scholar]
- 5.Kim EK, Ko KH, Oh KK, Kwak JY, You JK, Kim MJ, et al. Clinical application of the BI-RADS final assessment to breast sonography in conjunction with mammography. AJR Am J Roentgenol. 2008;190:1209–1215. doi: 10.2214/AJR.07.3259. [DOI] [PubMed] [Google Scholar]
- 6.Berg WA, Cosgrove DO, Dore CJ, Schafer FK, Svensson WE, Hooley RJ, et al. Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology. 2012;262:435–449. doi: 10.1148/radiol.11110640. [DOI] [PubMed] [Google Scholar]
- 7.Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012;265:59–69. doi: 10.1148/radiol.12120621. [DOI] [PubMed] [Google Scholar]
- 8.Barr RG, Zhang Z, Cormack JB, Mendelson EB, Berg WA. Probably benign lesions at screening breast US in a population with elevated risk: prevalence and rate of malignancy in the ACRIN 6666 trial. Radiology. 2013;269:701–712. doi: 10.1148/radiol.13122829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raza S, Chikarmane SA, Neilsen SS, Zorn LM, Birdwell RL. BI-RADS 3, 4, and 5 lesions: value of US in management. Follow-up and outcome. Radiology. 2008;248:773–781. doi: 10.1148/radiol.2483071786. [DOI] [PubMed] [Google Scholar]
- 10.Graf O, Helbich TH, Hopf G, Graf C, Sickles EA. Probably benign breast masses at US: is follow-up an acceptable alternative to biopsy? Radiology. 2007;244:87–93. doi: 10.1148/radiol.2441060258. [DOI] [PubMed] [Google Scholar]
- 11.Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–350. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 12.Raza S, Odulate A, Ong EM, Chikarmane S, Harston CW. Using realtime tissue elastography for breast lesion evaluation: our initial experience. J Ultrasound Med. 2010;29:551–563. doi: 10.7863/jum.2010.29.4.551. [DOI] [PubMed] [Google Scholar]
- 13.Cho N, Moon WK, Park JS, Cha JH, Jang M, Seong MH. Nonpalpable breast masses: evaluation by US elastography. Korean J Radiol. 2008;9:111–118. doi: 10.3348/kjr.2008.9.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi A, Cho N, Chang JM, Koo HR, La Yun B, Moon WK. Sonoelastography for 1,786 non-palpable breast masses: diagnostic value in the decision to biopsy. Eur Radiol. 2012;22:1033–1040. doi: 10.1007/s00330-011-2341-x. [DOI] [PubMed] [Google Scholar]
- 15.Cho N, Jang M, Lyou CY, Park JS, Choi HY, Moon WK. Distinguishing benign from malignant masses at breast US: combined US elastography and color Doppler US. Influence on radiologist accuracy. Radiology. 2012;262:80–90. doi: 10.1148/radiol.11110886. [DOI] [PubMed] [Google Scholar]
- 16.Cho N, Moon WK, Chang JM, Yi A, Koo HR, Park JS, et al. Sonoelastographic lesion stiffness: preoperative predictor of the presence of an invasive focus in nonpalpable DCIS diagnosed at US-guided needle biopsy. Eur Radiol. 2011;21:1618–1627. doi: 10.1007/s00330-011-2103-9. [DOI] [PubMed] [Google Scholar]
- 17.Athanasiou A, Tardivon A, Tanter M, Sigal-Zafrani B, Bercoff J, Deffieux T, et al. Breast lesions: quantitative elastography with supersonic shear imaging. Preliminary results. Radiology. 2010;256:297–303. doi: 10.1148/radiol.10090385. [DOI] [PubMed] [Google Scholar]
- 18.Chang JM, Moon WK, Cho N, Yi A, Koo HR, Han W, et al. Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat. 2011;129:89–97. doi: 10.1007/s10549-011-1627-7. [DOI] [PubMed] [Google Scholar]
- 19.Schafer FK, Hooley RJ, Ohlinger R, Hahne U, Madjar H, Svensson WE, et al. ShearWave™ Elastography BE1 multinational breast study: additional SWE™ features support potential to downgrade BI-RADS®-3 lesions. Ultraschall Med. 2013;34:254–259. doi: 10.1055/s-0033-1335523. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Chang JM, Cho N, Koo HR, Yi A, Kim SJ, et al. Practice guideline for the performance of breast ultrasound elastography. Ultrasonography. 2014;33:3–10. doi: 10.14366/usg.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Bohm-Velez M, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon WK, Myung JS, Lee YJ, Park IA, Noh DY, Im JG. US of ductal carcinoma in situ. Radiographics. 2002;22:269–280. doi: 10.1148/radiographics.22.2.g02mr16269. [DOI] [PubMed] [Google Scholar]


