Abstract
Cell therapy is a major discipline of regenerative medicine that has been continually growing over the last two decades. The aging of the population necessitates discovery of therapeutic innovations to combat debilitating disorders, such as stroke. Menstrual blood and Sertoli cells are two gender-specific sources of viable transplantable cells for stroke therapy. The use of autologous cells for the subacute phase of stroke offers practical clinical application. Menstrual blood cells are readily available, display proliferative capacity, pluripotency and angiogenic features, and, following transplantation in stroke models, have the ability to migrate to the infarct site, regulate the inflammatory response, secrete neurotrophic factors, and have the possibility to differentiate into neural lineage. Similarly, the testis-derived Sertoli cells secrete many growth and trophic factors, are highly immunosuppressive, and exert neuroprotective effects in animal models of neurological disorders. We highlight the practicality of experimental and clinical application of menstrual blood cells and Sertoli cells to treat stroke, from cell isolation and cryopreservation to administration.
Keywords: menstrual blood, Sertoli cells, autologous, ischemic stroke, regenerative
Introduction
The availability of stem cells has provided an alternative platform to investigate developmental processes of cell growth, proliferation, and differentiation. The use of stem cell for therapeutic application has been explored in the laboratory and reached limited clinical over the last decade for treating different brain diseases such as those characterized by neurodegeneration, inflammation, trauma and autoimmune alterations. Tissue sources of stem cells have included the fetus, teratocarcinoma cells, embryo, adult tissues such as bone marrow, umbilical cord, placenta, and menstrual blood, and recently skin fibroblasts that can be induced to display stemness properties, characterized by high proliferative and differentiation potential, as well as neurotrophic and immunomodulatory secretory function [1]. Stem cells that could circumvent the host immune response appear as the choice for transplantation therapy. Along this line, research efforts have entertained the idea of autologous transplantation. To this end, gender-specific donor cells have been explored, including menstrual blood-derived stem cells and testis-derived Sertoli cells. Both cells have been deemed as transplantable cells, which can de delivered directly into the brain or peripherally including intravenous or intra-arterial route of administration for treating neurological disorders.
Neurovascular diseases are the third leading cause of death in the United States, and the first cause of chronic disability [2,3,4-6]. Changes in lifestyles and aging, especially in developed countries, are contributors to the statistical increase of such diseases, especially stroke [7]. Despite these statistical findings, treatment historically has been limited. The only therapeutic technique approved to date is tissue plasminogen activator (tPA) and that treatment, in itself, has a very small time window (up to 3-4.5 hours after symptoms onset) [8, 9]. A report from 2008 estimated that only 1.8-2.1% of all stroke patients nationwide have been treated with tPA [10]. These staggering results suggest that an alternative therapeutic option, such as abrogating the secondary cell death [11-14], such as inflammation [15-18], must be explored for stroke patients. Embryonic and adult stem cells have been shown to afford therapeutic benefits against this stroke-mediated secondary cell death [19-22].
Laboratory data indicate that transplantation of menstrual blood cells improve functional outcomes in animal models of stroke. Migration to the site of injury, immunomodulation, and secretion of neurotrophic factors are the main attributes of these cells as a therapeutic product. Menstrual blood has been shown to demonstrate more immature phenotypes and behavior while preserving the adult stem cell safety characteristics, when compared to bone marrow cells [2-3,7-8,10,23,24]. Experimental studies have shown the benefits of menstrual blood cells as therapeutic tools with tissue repair and improvement of functionality in the heart, ischemic limbs, and the central nervous system [24-27].
In parallel to the menstrual blood cells, the male-derived testis-derived Sertoli cells have also been shown to improve functional recovery in animal models of neurological disorders. The main transplantable attributes of Sertoli cells consist of their astounding immunosuppressive properties, which aid in limiting graft rejection, and the many trophic factors that are beneficial in assisting the remodeling process. Laboratory studies documenting neuroprotective effects in parkinsonian rats [28,29] are only a few of the experiments that have demonstrated the potential use of Sertoli cells for therapeutic applications.
This review paper will characterize menstrual blood and Sertoli cells, discuss their mechanisms of repair in neurological disorders with emphasis in stroke, and present transplantable features of menstrual blood cells and Sertoli cells as autologous cell donors for personalized medicine.
Differences between male and female cells
While this paper primarily focuses on two sex-specific cell sources, namely menstrual blood cells from females and Sertoli cells from males, some sex-specific cell features need to be recognized. The first and most obvious difference between the sexes is the presence of the Y-chromosome in males. While much of it consists of repeats of the X-chromosome genes, there are male specific regions which code for 27 proteins not found on the X-chromosome (and hence not in females). Of considerable interest is the fact that 8 of these are expressed in the brain [30]. In conjunction with the absence of the paternal genomic imprint of the X-chromosome in males, this would also support the observation that there are intrinsic differences between male and female cells, implying that one cell (from one sex) is unlikely to ‘fit all’.
It is well known that males and females exhibit different susceptibilities to different disorders. Studies have implicated the influence of gender in stroke pathology. Gender differences in an animal model relating to stroke were first shown in 1974 [31], when it was demonstrated that the incidence of stroke in male spontaneously hypertensive rats was significantly greater than the incidence in females. Epidemiological studies in humans have shown a similar observation for the occurrence of stroke [32] and cardiovascular disease [33]. Females were found to possess a reduced incidence of stroke up to the age of 75, but an increased likelihood of death from myocardial infarction up to this age. Also, two sex-specific clinical trials for aspirin in the treatment of cardiovascular disease revealed that aspirin was protective against a first stroke in females but not males, while reducing the incidence of a first heart attack in males but not females. While another major difference between males and females is the presence of the gonadal hormones, this is clearly not the whole story, as the above observations are seen long after menopause and standardization of these hormones between the sexes, as well as neonatal and prepubescent advantages. The reproductive hormones such as estrogen and testosterone have been shown to influence cell survival and activity, since estrogen has been demonstrated to be protective, while testosterone exacerbates the hypoxic effects of the succinate dehydrogenase inhibitor, 3-nitropropionic acid, on the lateral striatal artery of rats [34]. They showed that castration had no effect, while ovariectomy or testosterone treatment potentiated the hypoxia. However, translation of the protective effects of estrogen to the clinic warrants further examination.
Although many studies have not taken into account the gender of cells used for culturing or transplanting or the gender of the recipient, a few studies have explored gender differences and possible mechanisms. Several reviews have recently been published [35,36] which reveal that male and female cells respond differently to specific insults with female cells generally being resistant, though this is both insult and source dependent. Female cells were found to undergo a caspase-dependent cell death via activation of caspase 9 and caspase 3, while male cells undergo a caspase-independent form of cell death via production of peroxynitrite ions and other free radicals, apoptosis inducing factor (AIF) and poly(ADP-ribose) polymerase (PARP) activation in response to ischemic insults. However, it is worth noting that while PARP and nitric oxide synthase (NOS) inhibitors would be expected to be protective in males, they were also found to be detrimental in females [37]. Even though PARP and AIF are equally active in females, they have been shown not to be involved in the ischemic response in females [38]. Conversely, caspase inhibitors were effective in reducing the ischemic response to a neonatal stroke in female but not male rats [39]. One possible mechanism of action for the neuroprotection seen in females may relate to astrocytic overexpression of P450 aromatase. This allows for the production of the neuroprotective steroid 17β-estradiol (from e.g. testosterone), as has been demonstrated using conditioned media and a P450 aromatase inhibitor [40].
Studies in rats clearly show that stroke causes immune dysfunction [41-43], however, these studies were all performed in males. This has also been shown to be true in female ovariectomized rats [44]. Immune function could be restored in these females by supplementation with estradiol or an estrogen receptor agonist. Estradiol has been shown to exert neuroprotection against experimental stroke via enhanced interleukin-1beta expression [45] or neurogenesis [46], altogether demonstrating alternative mechanisms independent of conventional gender influence. However, some animal models of postmenopausal females show a toxic effect of estrogen. It has been suggested that this may relate to the reduced expression of insulin-like growth factor (IGF) in the aged animals [47].
The behavior of stem cells also changes depending on gender. Mesenchymal stem cells (MSCs) derived from male mouse bone marrow have been shown to be more susceptible to hypoxia in vitro than those cells from females. Their cytokine expression was also found to be pro-inflammatory with greater tumor necrosis factor (TNF) and interleukin-6 (IL-6) levels than female cells, while the reverse was true for anti-inflammatory vascular endothelial growth factor (VEGF) [48]. Transplantation of female mouse MSCs into isolated rat hearts of a non-specified gender following ischemia were shown to promote more functional recovery and increased VEGF and lower TNF expression, when compared to transplanted male cells [49]. A similar shift from proinflammatory cytokines to anti-inflammatory and hematopoietic regulatory cytokines was seen when female bone marrow-derived mononuclear cells (MNCs) were transplanted into male atherosclerotic ApoE−/− mice, which coincided with atherosclerotic plaque reduction. Male cells were not effective. Additionally, female ApoE−/− mice had a higher rate of endogenous repair compared to male mice [50], but, following cell transplantation of either sex, neither exhibited any further atheroprotection [51]. Menstrual blood stem cells may also promote anti-inflammatory cytokines, since their addition to mixed lymphocyte cultures resulted in stimulation of IL-4 and inhibition of TNF after endotoxin stimulation [26] and the monthly shedding of the endometrium during menstruation supports a role in angiogenesis and tissue replacement. Sertoli cells have also been shown to secrete proangiogenic factors [52] as well as promote tolerance when cotransplanted [53]. These studies suggest that menstrual blood stem cells and Sertoli cells could both be beneficial via modulatory means.
The neurogenic potential of MSCs from 2 year old Rhesus monkeys was shown to be greater with female-derived compared with male-derived cells [54]. Neural stem cells from young and old rats displayed sexual dimorphism with respect to steroid receptors and neural fate. Cells from males tended to adopt an oligodendroglial or neuronal fate while female cells adopted an astrocytic fate [55]. Male cells also overexpressed aromatase and estrogen receptor β (ERβ), with estrogen receptor α(ERα) being predominant in females [55,56]. Aging led to a decreased expression of neural markers but a normalization of estrogen receptors at a higher level [55,56]. There is some debate whether Sertoli cells express ERα and ERβ, since humans have shown a preference towards ERα rather than ERβ [57], while negligible ERα expression suggests the opposite is true [58]. This holds true with baboons, which have shown higher ERβthan ERα [59]. They also were found to express aromatase [58,59]. There have been no reports on whether menstrual blood cells express these receptors, but it is clear that the endothelial cells of the endometrium primarily express ERβ while the perivascular cells express both receptors [60].
Transplantation of young and old male and female NSCs into young and old rats revealed a lack of cell survival in young rats of either sex, but enhanced neurogenesis, while grafted cells survived in older animals with either same sex young cells or opposite sex old cells [61]. From this, the sex (and age) of both the recipient and the donor are important and in some cases autologous (or same sex) transplants may be more effective, while in other instances allogenic (particularly opposite sex) should be considered. Although the potential effects of sexual dimorphism on NSCs have been recently reviewed [62], the influence of sex-specific cell sources, such as menstrual blood and Sertoli cells, in stem cell biology and therapy remains underexplored.
Characterization of donor cells for cell therapy
A. Transplantable properties of endometrial cells
It was over 30 years ago that the presence of stem cells in the endometrium was first described [63]. Based on the idea of monthly shedding of the superficial layers, cells with high proliferating capacities were detected in the tissue [64,65]. Contrary to the initial belief that the stem cells were exclusively found permanently in the basalis layer of the endometrium, stem cells have been discovered in the menstrual blood [65], which was subsequently confirmed [23,66,67]. Epithelial and stromal cells separated from the endometrium or from menstrual blood and cultured in vitro show clonogenicity and proliferative capacity. However, the epithelial cells soon lose part of their phenotypic markers and need a feeder layer to survive [65,68]. Stromal stem cells isolated from menstrual blood (MenSCs) were determined to have the capability to expand in vitro, and showed clonogenic properties and multipotentiality [23]. The MenSCs were shown to express markers of pluripotency, such as Oct-4, SSEA-4 and ckit, which are frequently found in more immature cell types, including the embryonic stem cells. Recently, analysis has been performed on the proliferative properties of human menstrual blood-derived cells [66], which displayed high proliferative rates and an immature phenotype, showing embryonic cell markers, and remained unaltered after 20 culture passages. The cells demonstrated resistance, though, since they could be processed up to 96 hours after collection. They also had high viability after processing and longevity, as some of the cultures could be subcultured for 47 times before senescence.
B. Transplantable properties of Sertoli cells
Sertoli cells isolated from the testis serve many functions, including the formation of the blood testis barrier [69]. Sertoli cells are part of the seminiferous tubules, in which spermatogenesis and germ cell mitosis take place. The gap junctions of the tubules, specifically those between the Sertoli cells and germ cells, aid in communication of the morphology of the sperm cells. Sertoli cells have been shown to augment engraftment of both allogenic and xenogenic cells and aid in the therapy for disorders such as Parkinson’s Disease, type 1 diabetes, and Huntington’s Disease [52,70-73].
Sertoli cells are known to express trophic factors and aid in tissue remodeling. They also express regulatory proteins and nutritive factors as well which assist in the development of the testis and stimulate the growth of germ cells [28,74-79]. Contributing to germ cell viability is essential in protecting against immune detection and possible rejection [52]. Their ability to provide immunogenic protection means that the testes are considered an immunologic “privileged” organ site [28]. Two alleged immunosuppressive factors thought to be released by Sertoli cells are the CD-95 ligand-mediated mechanism [80] and the Fas ligand (Fas-L) [72,80-82]. Sertoli cells also express GATA-4, which regulates gene expression and cellular differentiation, and Sox9 [83], which is essential for Sertoli cell differentiation [84-87] and identification [88]. It also expresses GDNF, which is thought to aid in the self-renewal of spermatogonial stem cells [89,90]. Several studies also indicate that Sertoli cells even secrete angiogenic factors [75,91] and stimulate neovascularization [52].
Experimental and clinical applications of cell therapy
A. Transplantation studies using endometrial cells
A murine model of Duchenne muscular dystrophy revealed that myoblasts injected with a combination of endometrial and menstrual blood cells facilitated the production of human dystrophin in the treated muscle [67]. In addition, differentiating menstrual blood-derived stromal cells in vitro were shown to result in spontaneously beating cardiomyocyte-like cells [24]. Despite cell engraftment and transdifferentiation into cardiac tissue, transplanted endometrial-derived cells into neural tissues did not show evidence of expressive differentiation [25]. Stromal-like menstrual blood stem cells when for CD117, a marker associated with high proliferation, migration and survival maintained expression of embryonic-like stem cell phenotypic markers, such as Oct4, SSEA-4 and Nanog, even when cultured up to 9 passages [92]. Furthermore, when added to cultured rat neurons exposed to a hypoxic insult, the menstrual blood cells exerted neuroprotection and when transplanted into a rat stroke model, functional tests, irrespective of the injection site, i.e. systemic or local administration into the striatum, led to improved neurological performance [25]. Analysis of the tissue revealed that some human cells migrated to other, non-injected areas in the rat brain in addition to the injection area, without signs of differentiation, suggesting that, at least in brain tissue, cell differentiation is not the main contributing mechanism of repair [25].
Endometrial-derived cells have been used in a Parkinson’s disease mouse model [27]. Endometrial-derived stromal cells were differentiated in vitro into dopaminergic-like cells, which expressed nestin and tyrosine hydroxylase (TH), an enzyme involved in dopamine synthesis. These cells were able to migrate to the substantia nigra and showed in vivo differentiation, having neural phenotype and expressing human TH, supporting the therapeutic potential of the cells to restore the functionality of the damaged tissue.
A clinical study evaluated the safety of endometrial derived stromal cell administration [93]. Intrathecal injections of 16 to 30 million cells were given to four patients with multiple sclerosis. One of the four patients was given an additional intravenous injection. Results showed no adverse events and functional stability was still evident at 12 months later [93].
Endometrium-derived cells present a strong angiogenic potential which is most likely related to the function of the cells in the endometrium, i.e. rapid proliferation and implantation of the embryo [94]. Using the cells associated to intelligent artificial films [95], it is envisioned that the angiogenic properties of endometrial-derived cells can be used to treat chronic limb ischemia patients and severe skin burns.
B. Transplantation studies using Sertoli cells
Sertoli cells have been shown to provide neuroprotection in 6-hydroxdopamine-induced hemiparkinsonism in rats [28,29] and to aid the survival of nonneural DA-secreting adrenal chromaffin cells with a very low CNS immune response [96,97]. Trophic factors have also proven to be critical in the recovery of behavioral deficits of Parkinson’s disease (PD) [79]. To better understand the mechanisms by which Sertoli cells work, cells were injected into male and female rats to test whether these cells were hormone dependent or if gender was a factor in their efficacy by injecting the cells into male and female rats [28]. The results showed that female hemiparkinsonian rats exhibited functional recovery that was parallel to the male rats. This disproves the notion that Sertoli cells are gender biased [96,97], and supports the notion that these cells’ therapeutic action is not dependent on testosterone as was previously reported in female diabetic rat model [72]. This study also suggests that Sertoli cells survived transplantation via a FAS ligand (FAS-L or CD-95)-induced mechanism [80,98].
Sertoli cells are also shown to aid in local immunosuppression after transplantation without additional immunosuppression [72,97,99] via the Fas-L pathway [72,80-82] by inducing apoptotic cell death of Fas-L-expressing T cells activated by graft antigens [80,81,100]. Indeed, both Sertoli cell allografts and xenografts survived for 2 months post transplantation without any systemic immunosuppression [29]. Because Sertoli cells also secrete nutritive, trophic, and regulatory proteins, such as sulfated glycoprotein-2, androgen binding protein, epidermal growth factor, transforming growth factor-a and -b, interleukin-like growth factor, basic fibroblast growth factor [75,79], and platelet-derived growth factor [101], these factors can also aid in graft survival.
As with menstrual blood cells, it would be ideal to isolate and bank Sertoli cells before an injury. The banked cells can then be thawed and expanded ex vivo then transplanted into the same patient (Figure 1). Banking the cells prior to ischemia would allow for the cells to be administered as off-the-shelf medications in the clinic.
Figure 1.
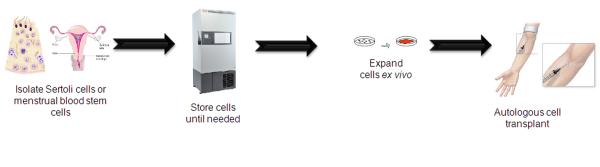
Separation and transplantation of autologous menstrual blood- and Sertoli-derived cells. Menstrual blood and Sertoli-derived cells can be harvested, expanded, and stored before a brain injury or disease onset. Immediately after injury or upon disease detection, the readily available autologous menstrual blood- and Sertoli-derived cells can be transplanted into the patient.
Personalized therapy: practical aspects
A. Endometrial cells and personalized medicine
Menstrual blood cells are a promising cell source for personalized cell therapy. Although stromal cells have low immunogenicity due to the lack of MHC class II expression [102] which enables allogenic application, autologous use is still preferred. Some advantages include safety, diminished risk of ethical conflicts, guaranteed lack of immunogenicity resulting in longer cell survival, and no induction of local inflammatory reaction. While cell-banking is already widely accessible for umbilical cord blood, only recently has it become available for menstrual blood cells. Women in child-bearing age may donate multiple samples of menstrual blood, enabling storage of large amounts of cells for future use, that may include expansion and differentiation into specific tissues for eventual transplantation [103].
B. Sertoli cells and personalized medicine
Despite the small window of pre-pubertal harvesting time and their inability to transdifferentiate into brain cells, Sertoli cells still hold promise for personalized medicine. Of note, Sertoli cells when preserved from juvenile male leukemia patients who are at risk for infertility can be used for treating acute lymphoblastic leukemia, whereby spermatogonial stem cells are transplanted back into the child, increasing the rate of post pubertal fertility [104]. A similar procedure may one day lead to stem cells taken from an individual prior to ischemic stroke then transplanted immediately post stroke.
The immunosuppressant benefits of Sertoli cells have also been found to lower blood glucose levels in diabetic mice [105], suggesting that it may be advantageous to perform a preventative transplantation of Sertoli cells in patients who are at risk of ischemic stroke [105].
Limitations of Stem Cell Donors
There are many limitations to stem cells including a severe deficit of donors, possible transfer of infection, age limitations, and the banking and storage methods. Menstrual blood stem cell banking is not an attractive proposal for women who overlook the advantages and believe it is an uncomfortable procedure and considered it to be “dirty”. Furthermore, the average age of menstruation for women is 14 to 45 years old; therefore they are in no rush to bank their cells. Finally, while the autologous nature of menstrual stem cells is beneficial for personalized application to the donor, their being autologous restricts them to the female population.
Finding the male equivalent to menstrual blood cells may prove to be a more arduous task. As with each type of stem cell there will always be limitations, with Sertoli cells’ constraints largely due to harvesting period and the lack of transdifferentiation potential of these cells [106-113]. Although markers exist for stem cells (in general), spermatogonial stem cells, and niche markers, it is still unknown if these cells directly mediate the functional recovery. There is no definite marker to localize and see the transdifferentiation that occurs on the brain when these cells are injected. Sertoli cells have been said to have Androgen binding protein as a marker, however, whether they contribute to neurogenesis directly is still unknown [114]. Furthermore, although studies have been conducted with xenografted Sertoli cells [29] and with the transplantation of Sertoli cells in female rats [28], there has been little suggestion for the possibility of autologous transplantation of Sertoli cells in males with neurodegenerative disease.
Conclusions
The rescue of the stroke brain is imperative for functional outcome and a great opportunity for cell therapy [5]. Stem cells may repair the stroke brain through modulation of the activated immune system and secretion of trophic factors [17]. Although cell differentiation is observed in the experimental setting, its importance to the final therapeutic outcome has been challenged. Utilizing the myriad of therapeutic molecules, including anti-inflammatory and immunosuppressive factors, for cell-based therapy is an attractive treatment for stroke. Cryopreservation of autologous cells may be a prudent strategy to those patients at risk of being affected by stroke. Despite the potential challenges still to be resolved, menstrual blood and Sertoli cells represent innovative therapeutic tools for stroke therapy.
Acknowledgements
CVB is funded by the James and Esther King Biomedical Research Foundation 1KG01-33966 and NIH 1R01NS071956-01.
Footnotes
Disclosures: CVB and PRS serve as consultants, and PRS is a co-founder of Saneron-CCEL Therapeutics, Inc., and CVB, PRS, and JGA have a patent application in this area, owned jointly by Cryo-Cell International, Inc. and Saneron-CCEL Therapeutics, Inc. Cryo-Cell International, Inc. provided the foundational menstrual stem cell technology in the patent applications of M. A. Walton and JGA wholly owned by Cryo-Cell International, Inc.
References
- 1.Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136–1145. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu J, Kenneth D, Kochanek MA, Sherry L, Murphy BS. Deaths, final data for 2007. Natl Vital Stat Rep. 2010;58:1–134. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Prevalence of disabilities and associated health conditions among adults, United States. M.M.W.R.: Morb Mortal Wkly Rep. 1999;50:120–125. (1999) [PubMed] [Google Scholar]
- 4.Richard Green A, Odergen T, Ashwood T. Animal models of stroke: do they have value for discovering neuroprotective agents? Trends Pharmacol Sci. 2003;24:402–408. doi: 10.1016/S0165-6147(03)00192-5. [DOI] [PubMed] [Google Scholar]
- 5.Chavez JC, Hurko O, Barone FC, Feuerstein GZ. Pharmacologic interventions for stroke: looking beyond the thrombolysis time window into the penumbra with biomarkers, not a stopwatch. Stroke. 2010;40:e558–563. doi: 10.1161/STROKEAHA.109.559914. [DOI] [PubMed] [Google Scholar]
- 6.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 7.Ovbiagele B, Nguyen-Huynh MN. Stroke Epidemiology: Advancing Our Understanding of Disease Mechanism and Therapy. Neurotherapeutics. 2011;8:319–329. doi: 10.1007/s13311-011-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study Group: Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 9.Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert Rev Neurothe. 2008;8:1193–1201. doi: 10.1586/14737175.8.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use, ICD-9 codes substantially underestimate. Stroke. 2008;39:924–928. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 11.Kriz J. Inflammation in ischemic brain injury, timing is important. Crit Rev Neurobiol. 2006;18:145–157. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- 12.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emsley CA, Smith CJ, Tyrrell PJ, Hopkins SJ. Inflammation in acute ischemic stroke and its relevance to stroke critical care. Neurocrit Care. 2008;9:125–138. doi: 10.1007/s12028-007-9035-x. [DOI] [PubMed] [Google Scholar]
- 14.Takano T, Oberbeim N, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke. 2009;40:S8–12. doi: 10.1161/STROKEAHA.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park DH, Eve DJ, Musso J, 3rd, et al. Inflammation and Stem Cell Migration to the Injured Brain in Higher Organisms. Stem Cells Dev. 2009;18:693–701. doi: 10.1089/scd.2009.0008. [DOI] [PubMed] [Google Scholar]
- 16.Bai L, Lennon DP, Eaton V, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucherie C, Hermans E. Adult stem cell therapies for neurological disorders: benefits beyond neuronal replacement? J Neurosci Res. 2009;87:1509–1521. doi: 10.1002/jnr.21970. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara Y, Tanaka N, Ishida O, et al. Intravenously injected neural progenitor cells of transgenic rats can migrate to the injured spinal cord and differentiate into neurons, astrocytes and oligodendrocytes. Neurosci Lett. 2004;366:287–291. doi: 10.1016/j.neulet.2004.05.080. [DOI] [PubMed] [Google Scholar]
- 19.Hill WD, Hess DC, Martin-Studdard A, et al. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- 20.Daar AS, Bhatt A, Court E, Singer PA. Stem cell research and transplantation: Science leading ethics. Transpla Proc. 2004;36:2504–2506. doi: 10.1016/j.transproceed.2004.08.129. [DOI] [PubMed] [Google Scholar]
- 21.Takagi Y, Nishimura M, Morizane A, et al. Survival and differentiation of neural progenitor cells derived from embryonic cells and transplanted into ischemic brain. J Neurosurg. 2004;103:304–310. doi: 10.3171/jns.2005.103.2.0304. [DOI] [PubMed] [Google Scholar]
- 22.Ikegame Y, Yamashita K, Hayashi S, et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy. 2011;13:675–685. doi: 10.3109/14653249.2010.549122. [DOI] [PubMed] [Google Scholar]
- 23.Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent Menstrual Blood Stromal Stem Cells: Isolation, Characterization and Differentiation. Cell Transplant. 2008;17:303–311. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- 24.Hida N, Nishiyama N, Miyoshi S, et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26:1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- 25.Borlongan CV, Kaneko Y, Maki M, et al. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19:439–451. doi: 10.1089/scd.2009.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy MP, Wang H, Patel AN, et al. Allogeneic endometrial regenerative cells, an “Off the shelf solution” for critical limb ischemia? J Transl Med. 2008;6:45. doi: 10.1186/1479-5876-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff EF, Gao XB, Andrews ZB, Du H, Elsworth JD, Taylor HS. Endometrial stem cell transplantation restores dopamine production in a Parkinson’s disease model. J Cell Mol Med. 2011;15:747–755. doi: 10.1111/j.1582-4934.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borlongan CV, Cameron DF, Saporta S, Sandberg PR. Intracerebral Transplantation of Testis-Derived SC Promotes Functional Recovery in Female Rats with 6-Hydroxydopamine-Induced Hemiparkinsonism. Exp Neurol. 1997;148:388–392. doi: 10.1006/exnr.1997.6513. [DOI] [PubMed] [Google Scholar]
- 29.Saporta S, Cameron DF, Borlongan CV, Sanberg PR. Survival of Rat and Porcine Sertoli Cell Transplants in the Rat Striatum without Cyclosporine-A Immunosuppression. Exp Neurol. 1997;146:299–304. doi: 10.1006/exnr.1997.6493. [DOI] [PubMed] [Google Scholar]
- 30.Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- 31.Yamori Y, Nagaoka A, Okamoto K. Importance of genetic factors in stroke: an evidence obtained by selective breeding of stroke-prone and stroke-resistant SHR. Circ J. 1974;38:1095–1100. doi: 10.1253/jcj.38.1095. [DOI] [PubMed] [Google Scholar]
- 32.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: Results from an international collaboration. International stroke incidence Collaboration. Stroke. 1997;28:491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 33.Bairey Merz CN, Mark S, Boyan BD, et al. Proceedings from the Scientific Symposium: Sex differences in cardiovascular disease and implications for therapies. J Womens Health. 2010;19:1059–1072. doi: 10.1089/jwh.2009.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishino H, Nakajima K, Kumazaki M, et al. Estrogen protects against while testosterone exacerbates vulnerability of the lateral striatal artery to chemical hypoxia by 3-nitropropionic acid. Neurosci Res. 1998;30:303–312. doi: 10.1016/s0168-0102(98)00010-8. [DOI] [PubMed] [Google Scholar]
- 35.Herson PS, Hurn PD. Gender and the injured brain. Prog Brain Res. 2010;186:177–187. doi: 10.1016/B978-0-444-53630-3.00012-9. [DOI] [PubMed] [Google Scholar]
- 36.Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 38.Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD. Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol. 2009;217:210–218. doi: 10.1016/j.expneurol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renolleau S, Fau S, Goyenvalle C, et al. Specific caspase inhibitor QVD-OPh prevents neonatal stroke in P7 rat: a role for gender. J Neurochem. 2007;100:1062–1071. doi: 10.1111/j.1471-4159.2006.04269.x. (2007) [DOI] [PubMed] [Google Scholar]
- 40.Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. J Cereb Blood Flow Metab. 2007;27:135–141. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- 41.Ajmo CT, Jr, Vernon DO, Collier L, et al. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 43.Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang B, Subramanian S, Dziennis S, et al. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J Immunol. 2010;184:4087–4094. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiappetta O, Gliozzi M, Siviglia E, et al. Evidence to implicate early modulation of interleukin-1beta expression in the neuroprotection afforded by 17beta-estradiol in male rats undergone transient middle cerebral artery occlusion. Int Rev Neurobiol. 2007;82:357–372. doi: 10.1016/S0074-7742(07)82019-8. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Siegel M, Yuan M, et al. Estrogen enhances neurogenesis and behavioral recovery after stroke. J Cereb Blood Flow Metab. 2011;31:413–425. doi: 10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 2010;30:6852–6861. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crisostomo PR, Wang M, Herring CM, et al. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1) J Mol Cell Cardiol a. 2007;42:142–149. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crisostomo PR, Markel TA, Wang M, Lahm T, Lillemoe KD, Meldrum DR. In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery b. 2007;142:215–221. doi: 10.1016/j.surg.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Zenovich AG, Panoskaltsis-Mortari A, Caron GJ, et al. Sex-based differences in vascular repair with bone marrow cell therapy: relevance of regulatory and Th2-type cytokines. Transplant Proc. 2008;40:641–643. doi: 10.1016/j.transproceed.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 51.Nelson WD, Zenovich AG, Ott HC, et al. Sex-dependent attenuation of plaque growth after treatment with bone marrow mononuclear cells. Circ Res. 2007;101:1319–1327. doi: 10.1161/CIRCRESAHA.107.155564. [DOI] [PubMed] [Google Scholar]
- 52.Golat BT, Cameron DF. Sertoli cells enhance formation of capillary-like structures in vitro. Cell Transplant. 2008;17:1135–1144. doi: 10.3727/096368908787236512. [DOI] [PubMed] [Google Scholar]
- 53.Shamekh R, El-Badri NS, Saporta S, Pascual C, Sanberg PR, Cameron DF. Sertoli cells induce systemic donor-specific tolerance in xenogenic transplantation model. Cell Transplant. 2006;15:45–53. doi: 10.3727/000000006783982205. [DOI] [PubMed] [Google Scholar]
- 54.Yuan J, Yu J-X, Ge J. Sexual dimorphism on the neurogenic potential of rhesus monkeys mesenchymal stem cells. Biochem Biophys Res Comm. 2010;396:394–400. doi: 10.1016/j.bbrc.2010.04.103. [DOI] [PubMed] [Google Scholar]
- 55.Waldron J, McCourty A, Lecanu L. Aging differentially affects male and female neural stem cell neurogenic properties. Stem Cells Cloning: Adv Appl a. 2010;3:119–127. doi: 10.2147/SCCAA.S13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waldron J, McCourty A, Lecanu L. Neural stem cell sex dimorphism in aromatase (CYP19) expression: a basis for differential neural fate. Stem Cells Cloning: Adv Appl b. 2010;3:175–182. doi: 10.2147/SCCAA.S15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavaco JE, Laurentino SS, Barros A, Sousa M, Socorro S. Estrogen receptors alpha and beta in human testis: both isoforms are expressed. Syst Biol Reprod Med. 2009;55:137–144. doi: 10.3109/19396360902855733. [DOI] [PubMed] [Google Scholar]
- 58.Berensztein EB, Baquedano MS, Gonzalez CR, et al. Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatr Res. 2006;60:740–744. doi: 10.1203/01.pdr.0000246072.04663.bb. [DOI] [PubMed] [Google Scholar]
- 59.Bonagura TW, Zhou H, Babischkin JS, Pepe GJ, Albrecht ED. Expression of P-450 aromatase, estrogen receptor α and β, and α-inhibin in the fetal baboon testis after estrogen suppression during the second half of gestation. Endocrine. 2011;39:75–82. doi: 10.1007/s12020-010-9414-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Critchley HO, Brenner RM, Henderson TA, et al. Estrogen receptor beta, but not estrogen receptor alpha, is present in the vascular endothelium of the human and nonhuman primate endometrium. J Clin Endocrinol Metab. 2001;86:1370–1378. doi: 10.1210/jcem.86.3.7317. [DOI] [PubMed] [Google Scholar]
- 61.Waldron J, Lecanu L. Age and sex differences in neural stem cell transplantation: a descriptive study rats. Stem Cells Cloning: Adv Appl. 2011;24:25–37. doi: 10.2147/SCCAA.S18653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lecanu L. Sex, the underestimated potential determining factor in brain tissue repair strategy. Stem Cells Dev. 2011;20:2031–2035. doi: 10.1089/scd.2011.0188. [DOI] [PubMed] [Google Scholar]
- 63.Prianishnikov VA. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception. 1978;18:213–223. doi: 10.1016/s0010-7824(78)80015-8. [DOI] [PubMed] [Google Scholar]
- 64.Padykula HA. Regeneration in the primate uterus, the role of stem cells. Ann NY Acad Sci. 1991;622:47–52. doi: 10.1111/j.1749-6632.1991.tb37849.x. [DOI] [PubMed] [Google Scholar]
- 65.Meng X, Ichim TE, Zhong J, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allickson JG, Sanchez A, Yeflmenko N, Borlongan CV, Sanberg PR. Recent Studies Assessing the Proliferative Capability of a Novel Adult Stem Cell Identified in Menstrual Blood. Open Stem Cell J. 2011;3:4–10. doi: 10.2174/1876893801103010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui CH, Uyama T, Miyado K, et al. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol Biol Cell. 2007;18:1586–1594. doi: 10.1091/mbc.E06-09-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reproduction. 2004;70:1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 69.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin Cell Dev Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 70.Emerich DF, Hemendinger R, Halberstadt CR. The Testicular-Derived Sertoli Cell: Cellular Immunoscience to Enable Transplantation. Cell Transplant. 2003;12:335–349. doi: 10.3727/000000003108746894. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez AI, Willing AE, Saporta S, Cameron DF, Sanburg PR. Effects of sertoli cell transplants in a 3-nitropropionic acid model of early Huntington’s disease: A preliminary study. Neurotox Res. 2003;5:443–450. doi: 10.1007/BF03033174. [DOI] [PubMed] [Google Scholar]
- 72.Selawry HP, Cameron DF. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant. 1993;2:123–129. doi: 10.1177/096368979300200206. [DOI] [PubMed] [Google Scholar]
- 73.Shamekh R, Newcomb J, Mallery J, Cassady CJ, Saporta S, Cameron DF. Survival of rat or mouse ventral mesencephalon neurons after co-transplantation with rat Sertoli cells in the mouse striatum. Cell Transplant. 2005;14:551–564. doi: 10.3727/000000005783982747. [DOI] [PubMed] [Google Scholar]
- 74.Flokman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 75.Griswold MD. Protein secretion by Sertoli cells. In: Russell LD, Griswold MD, editors. General considerations. The Sertoli Cell Cache River Press; Clearwater: 1993. pp. 195–200. [Google Scholar]
- 76.Niederberger CS, Shubhada S, Kim SJ, Lamb DJ. Paracrine factors and the regulation of spermatogenesis. World J Urol. 1993;11:120–128. doi: 10.1007/BF00182039. [DOI] [PubMed] [Google Scholar]
- 77.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 78.Skinner M. Sertoli cell secreted regulatory factors. In: Skinner M, Griswold M, editors. Sertoli cell biology. Elsevier Academic Press; San Diego: 2005. pp. 95–120. [Google Scholar]
- 79.Skinner MK. Secretion of growth factors and other regulatory factors. In: Russell LD, Griswold MD, editors. General considerations. The Sertoli Cell Cache River Press; Clearwater: 2003. pp. 237–247. [Google Scholar]
- 80.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD-95 ligand in preventing graft rejection. Nature. 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 81.French LE, Hahne M, Viard I, et al. Fas and Fas ligand in embryos and adult mice: Ligand expression in several immuneprivileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol. 1996;133:335–343. doi: 10.1083/jcb.133.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanberg PR, Saporta S, Borlongan CV, Othberg AL, Allen RC, Cameron DF. The testis-derived cultured Sertoli cell as a natural Fas-L secreting cell for immunosuppressive cellular therapy. Cell Transplant. 1997;62:191–193. doi: 10.1177/096368979700600213. [DOI] [PubMed] [Google Scholar]
- 83.Kojima Y, Hayashi Y, Mizuno K, et al. Up-regulation of SOX9 in human sex-determining region on the Y chromosome (SRY)-negative XX males. Clin Endocrinol. 2008;68:791–799. doi: 10.1111/j.1365-2265.2007.03101.x. [DOI] [PubMed] [Google Scholar]
- 84.Frojdman K, Harley VR, Pelliniemi LJ. Sox9 protein in rat Sertoli cells is age and stage dependent. Histochem Cell Biol. 2000;113:31–36. doi: 10.1007/s004180050004. [DOI] [PubMed] [Google Scholar]
- 85.Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- 86.Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14:62–68. doi: 10.1038/ng0996-62. [DOI] [PubMed] [Google Scholar]
- 87.Bockers TM, Nieschlag E, Kreutz MR, Bergmann M. Localization of follicle-stimulating hormone (FSH) immunoreactivity and hormone receptor mRNA in testicular tissue of infertile men. Cell Tissue Res. 1994;278:595–600. doi: 10.1007/BF00331379. [DOI] [PubMed] [Google Scholar]
- 88.Hemendinger RA, Gores P, Blacksten L, Harley V, Halberstadt C. Identification of a specific Sertoli cell marker, Sox9, for use in transplantation. Cell Transplant. 2002;11:499–505. [PubMed] [Google Scholar]
- 89.Davidoff MS, Middendorff R, Koeva Y, Pusch W, Jezek D, Muller D. Glial cell line-derived neurotrophic factor (GDNF) and its receptors GFRalpha-1 and GFRal pha-2 in the human testis. Ital J Anat Embryol. 2001;106:173–180. [PubMed] [Google Scholar]
- 90.Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol. 2008;288:95–103. doi: 10.1016/j.mce.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dufour JM, Rajotte RV, Korbutt GS, Emerich DF. Harnessing the immunomodulatory properties of sertoli cells to enable xenotransplantation in type I diabetes. Immunol Invest. 2003;32:275–297. doi: 10.1081/imm-120025106. [DOI] [PubMed] [Google Scholar]
- 92.Cho NH, Park YK, Kim YT, Yang H, Kim SK. Lifetime expression of stem cell markers in the uterine endometrium. Fertil Steril. 2004;81:403–407. doi: 10.1016/j.fertnstert.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 93.Zhong Z, Patel AN, Ichim TE, et al. Feasibility investigation of allogeneic endometrial regenerative cells. J Transl Med. 2009;7:15. doi: 10.1186/1479-5876-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fan X, Krieg S, Kuo CJ, et al. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008;22:3571–3580. doi: 10.1096/fj.08-111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Drago H, Marin GH, Sturla F, et al. The next generation of burns treatment, intelligent films and matrix, controlled enzymatic debridement, and adult stem cells. Transplant Proc. 2010;42:345–349. doi: 10.1016/j.transproceed.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 96.Sanberg PR, Borlongan CV, Saporta S, Cameron DF. Sertoli cells: An alternative cell source for neural transplantation in Parkinson’s disease. Exper Neurol. 1995;135:169. [Google Scholar]
- 97.Sanberg PR, Borlongan CV, Saporta S, Cameron DF. Testis-derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat Biotechnol. 1996;14:1692–1695. doi: 10.1038/nbt1296-1692. [DOI] [PubMed] [Google Scholar]
- 98.Lau HT, Yu M, Fontana A, Stoeckert CJ., Jr Prevention of islet rejection with engineered myoblast expressing FasL in mice. Science. 1996;273:109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 99.Sanberg PR, Othberg AI, Borlongan CV, et al. Transplantation of testis-derived Sertoli cells into the mammalian brain. Tranplant Proc. 1997;29:1926–1928. doi: 10.1016/s0041-1345(97)00164-4. [DOI] [PubMed] [Google Scholar]
- 100.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. FAS ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 101.Gnessi LA, Emidi EA, Jannini E, et al. Testicular development involves the spatiotemporal control of PDGFs and PDGF receptors gene expression and action. J Cell Biol. 1995;131:1105–1121. doi: 10.1083/jcb.131.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 103.Zhang MJ, Liu B, Xia W, Sun ZY, Lu KH. Could cells from menstrual blood be a new source for cell-based therapies? Med Hypotheses. 2009;72:252–254. doi: 10.1016/j.mehy.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 104.Nurmio M, Keros V, Lähteenmäki P, Salmi T, Kallajoki M, Jahnukainen K. Effect of childhood acute lymphoblastic leukemia therapy on spermatogonia populations and future fertility. J Clin Endocrinol Metab. 2009;94:2119–2122. doi: 10.1210/jc.2009-0060. [DOI] [PubMed] [Google Scholar]
- 105.Halley K, Dyson EL, Kaur G, et al. Delivery of a therapeutic protein by immune-privileged Sertoli cells. Cell Transplant. 2010;19:1645–1657. doi: 10.3727/096368910X516628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Izadyar F, Spierenberg GT, Creemers LB, den Ouden K, de Rooij DG. Isolation and purification of type A spermatogonia from the bovine testis. Reproduction. 2002;124:85–94. [PubMed] [Google Scholar]
- 107.Ryser S, Glauser D, Vigier M, et al. Gene expression profiling of rat spermatogonial and Sertoli cells reveals signaling pathways from stem cells to niche and testicular cancer cells to surrounding stroma. BMC Genomics. 2011;12:29. doi: 10.1186/1471-2164-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003;69:412–420. doi: 10.1095/biolreprod.103.016519. [DOI] [PubMed] [Google Scholar]
- 109.Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31:461–472. doi: 10.1054/tice.1999.0060. [DOI] [PubMed] [Google Scholar]
- 110.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc Nat Acad Sci USA. 2001;98:6186–6191. doi: 10.1073/pnas.111158198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McLean DJ, Friel PJ, Johnston DS, Griswold MD. Characterization of spermatogonial stem cell maturation and differentiation in neonatal mice. Biol Reprod. 2003;69:2085–2091. doi: 10.1095/biolreprod.103.017020. [DOI] [PubMed] [Google Scholar]
- 112.Yoshida S, Sukeno M, Nakagawa K, et al. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 113.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 114.Gunsalus GL, Larrea F, Musto NA, Becker PR, Mather JP, Bardin CW. Androgen binding protein as a marker for sertoli cell function. J Steroid Biochem. 1981;15:99–106. doi: 10.1016/0022-4731(81)90263-6. [DOI] [PubMed] [Google Scholar]


