Background: A four-gene operon, acsFII-ho2-hemN-desF, is most highly expressed under microoxic conditions.
Results: A chlR mutant cannot express this operon, and ChlR forms homodimers ligating a [4Fe-4S] cluster.
Conclusion: ChlR is a transcription activator that utilizes an oxygen-labile [4Fe-4S] cluster to sense oxygen.
Significance: ChlR is a simple regulatory element that could facilitate expression of O2-sensitive proteins.
Keywords: Cyanobacteria, Gene Expression, Iron-Sulfur Protein, Transcription Regulation, Transcriptomics
Abstract
Synechococcus sp. PCC 7002 and many other cyanobacteria have two genes that encode key enzymes involved in chlorophyll a, biliverdin, and heme biosynthesis: acsFI/acsFII, ho1/ho2, and hemF/hemN. Under atmospheric O2 levels, AcsFI synthesizes 3,8-divinyl protochlorophyllide from Mg-protoporphyrin IX monomethyl ester, Ho1 oxidatively cleaves heme to form biliverdin, and HemF oxidizes coproporphyrinogen III to protoporphyrinogen IX. Under microoxic conditions, another set of genes directs the synthesis of alternative enzymes AcsFII, Ho2, and HemN. In Synechococcus sp. PCC 7002, open reading frame SynPCC7002_A1993 encodes a MarR family transcriptional regulator, which is located immediately upstream from the operon comprising acsFII, ho2, hemN, and desF (the latter encodes a putative fatty acid desaturase). Deletion and complementation analyses showed that this gene, denoted chlR, is a transcriptional activator that is essential for transcription of the acsFII-ho2-hemN-desF operon under microoxic conditions. Global transcriptome analyses showed that ChlR controls the expression of only these four genes. Co-expression of chlR with a yfp reporter gene under the control of the acsFII promoter from Synechocystis sp. PCC 6803 in Escherichia coli demonstrated that no other cyanobacterium-specific components are required for proper functioning of this regulatory circuit. A combination of analytical methods and Mössbauer and EPR spectroscopies showed that reconstituted, recombinant ChlR forms homodimers that harbor one oxygen-sensitive [4Fe-4S] cluster. We conclude that ChlR is a transcriptional activator that uses a [4Fe-4S] cluster to sense O2 levels and thereby control the expression of the acsFII-ho2-hemN-desF operon.
Introduction
Microorganisms acclimate continuously to changes in their physicochemical environments, including changes in nutrient availability, energy sources, salinity, pH, and temperature. Light is the energy source for phototrophic organisms; and thus, light wavelength and total irradiance are usually among the most important environmental factors for phototrophic organisms. Cyanobacteria perform oxygenic photosynthesis; and therefore, the supply of reducing equivalents generated by the photosynthetic apparatus increases with increasing irradiance, but the oxygen levels inside cells as well as in the immediate micro-environment can fluctuate significantly because of photosystem II activity and changes in respiratory oxygen uptake (1). Thus, cyanobacteria can rapidly acclimate to diurnal shifts in light availability, to rapidly changing irradiance levels throughout the day, and to the accompanying changes in oxygen levels that result. Adjustments can be made by several mechanisms, including changes in gene expression; protein maturation, assembly and stability; post-translational modifications of enzymes; and even substrate availability for O2-dependent enzymes.
For the model cyanobacterium Synechococcus sp. PCC 7002 (hereafter Synechococcus 7002), the impact of physicochemical parameters has been extensively studied using global systems biological approaches, including transcriptomics, proteomics, and metabolomics (2–6). Some changes in culture conditions, such as dark incubation, cause dramatic changes in the transcriptome (2), whereas other conditions, such as limitation for nutrients, cause more specific and limited short term responses (3, 4). Large changes in transcript levels for the acsFII-ho2-hemN-desF operon, which encodes key enzymes required for chlorophyll a, phycocyanobilin, heme, and lipid biosynthesis, were observed in response to changes in oxygen (2). A similar gene cluster comprising the open reading frames (ORFs) sll1874 (acsFII/chlAII), sll1875 (ho2), and sll1876 (hemN) occurs in Synechocystis sp. PCC 6803 (hereafter Synechocystis 6803), and transcript levels for these three genes also coordinately increase under microoxic conditions (7).
The acsFII, ho2, and hemN genes encode important enzymes that are involved in pigment biosynthesis: Mg-protoporphyrin monomethyl ester oxidative ring cyclase, heme oxygenase, and coproporphyrinogen III oxidase, respectively. Many cyanobacterial genomes encode two genes that produce alternative enzymes for these functions. For example, in Synechocystis 6803, there are two Mg-protoporphyrin monomethyl ester oxidative ring cyclases, AcsFI/ChlAI (sll1214) and AcsFII/ChlAII (sll1874), respectively. AcsFI/ChlAI catalyzes the formation of the isocyclic ring of chlorophyll a (converting Mg-protoporphyrin IX monomethyl ester into 3,8-divinyl protochlorophyllide) under atmospheric oxygen levels, whereas under microoxic growth conditions, the same reaction is mainly performed by AcsFII/ChlAII (7). Interestingly, both enzymes are monooxygenases and require molecular O2 as a substrate. These enzymes are unrelated to Mg-protoporphyrin monomethyl ester oxidative ring cyclases of the BchE type, which are O2-independent enzymes of the radical S-adenosylmethionine superfamily that use water as the oxygen donor for formation of the 131-oxo group of (bacterio) chlorophylls and are widely distributed in anoxygenic phototrophs (8–11).
Cyanobacteria as well as rhodophyte, glaucophyte, and cryptomonad algae use phycobiliproteins as major antenna pigments for photosynthesis (12, 13). Because phycobiliproteins have numerous linear tetrapyrrole (phycobilin) chromophores, these organisms are critically dependent on heme oxygenase activity for the production of the bilin chromophores of the light-harvesting phycobiliproteins. Synechocystis 6803 has two heme oxygenases, Ho1 (sll1184) and Ho2 (sll1875), which can oxidatively cleave the heme macrocycle in a reaction that requires both O2 and reducing equivalents and which produces carbon monoxide and biliverdin, the precursor of all linear tetrapyrrole pigments (14). Like Ho1 of Synechocystis 6803 and the heme oxygenases of other organisms, Ho2 also requires molecular oxygen for its catalytic activity (15). Under microoxic conditions and especially at high irradiance levels, Ho2 is the major enzyme cleaving heme to form biliverdin. Ho1 catalyzes the same reaction under atmospheric O2 levels (16, 17).
The third gene of the cluster expressed under microoxic conditions, hemN (sll1876), encodes an oxygen-independent coproporphyrinogen III oxidase (HemN type), which belongs to the radical S-adenosylmethionine protein superfamily (18). It harbors an oxygen-sensitive [4Fe-4S] cluster and requires S-adenosylmethionine for catalysis (19, 20). Besides hemN, the Synechocystis 6803 genome includes a gene for an oxygen-dependent coproporphyrinogen III oxidase (HemF, sll1185), which is a monooxygenase with a binuclear iron center (21, 22). In Synechocystis 6803, hemN has been shown to be induced under microoxic conditions, but hemF is required for growth at atmospheric O2 levels (23). The role of a second hemN-like gene (sll1917) in Synechocystis 6803 remains unclear (23).
A microarray study in Synechocystis 6803 showed that the psbA1 gene, encoding an alternative D1 subunit of photosystem II, and the acsFII-ho2-hemN operon were the only four genes for which transcript levels increased substantially under microoxic conditions (24). Recently, a MarR-type transcriptional activator (sll1512) that apparently controls the expression of these four genes was described (25).
While Aoki et al. (25) were studying the product of sll1512, we identified a similar transcriptional regulator, the product of ORF SYNPCC7002_A1993 in Synechococcus 7002. In the studies presented here, we show that the product of this ORF, ChlR, is a transcription activator that controls the expression of a single operon encoding four genes in Synechococcus 7002. Additionally, we show that this transcription factor activates transcription from the acsFII promoter of Synechocystis 6803 in the absence of any other cyanobacterium-specific factors in Escherichia coli. Finally, we show that this transcription activator harbors a single oxygen-sensitive [4Fe-4S] cluster per homodimer that acts as the O2 sensory prosthetic group.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Culture Conditions
Synechococcus sp. strain PCC 7002 wild-type and mutant strains were maintained in liquid culture and on 1.5% agar plates in medium A supplemented with 1 mg of NaNO3 ml−1 (designated as medium A+) as described previously (2, 26). Cultures of the SynPCC7002_A1993 (ChlR) overexpression strain (under control of an ammonia-repressible promoter) that were used for RNA extractions were grown in a HEPES-buffered A medium (25 mm HEPES, pH 8.0 replaced 8.3 mm Tris-HCl, pH 8.2) containing either 12 mm NaNO3 or 10 mm NH4Cl (4). Liquid cultures were grown in tubes containing medium (25 ml) at 38 °C with continuous irradiation with 250 μmol of photons m−2 s−1. The cultures were sparged with either 1% (v/v) CO2 in air (standard conditions) or 1% (v/v) CO2 in N2 (microoxic conditions). The following antibiotic concentrations were added to the medium when appropriate: 50 μg ml−1 for spectinomycin and/or 20 μg ml−1 for gentamycin. Cultures for growth rate determination were cultivated without antibiotics, whereas cultures for RNA analyses were grown in the presence of the respective antibiotics. Cell growth was monitored by measuring the optical density at 730 nm (OD730 nm; 1.0 OD730 nm = 1.0 ± 0.2 × 108 cells ml−1) with a Genesys 10 spectrophotometer (ThermoSpectronic, Rochester, NY). Cultures for RNA analyses were inoculated at an OD730 nm between 0.05 and 0.1 from precultures that had been grown under the same conditions. When these cultures reached an OD730 nm of 0.7, three independently grown, replicate cultures were pooled. Cells derived from 25-ml aliquots of the cultures were rapidly centrifuged (5 min, 5000 × g, 4 °C), and the cell pellets were rapidly frozen in liquid nitrogen and stored at −80 °C until required.
Inactivation of chlR and Construction of Expression Systems
To inactivate the chlR (SynPCC7002_A1993) gene of Synechococcus 7002, ∼1000-bp regions immediately upstream and downstream of chlR were amplified by PCR with primers 1 and 2 for the upstream sequence and primers 3 and 4 for the downstream sequence (see Table 1 for oligonucleotide sequences). Oligonucleotides 2 and 3 introduced EcoRV sites 2 bases downstream from the start codon and 6 bases upstream of the stop codon. The PCR products for the upstream and downstream flanking regions were digested with EcoRV, and the aadA gene conferring streptomycin and spectinomycin resistance was excised as a 1091-bp Eco53kI fragment from plasmid pSRA2 (plasmids used and constructed in this study are listed in Table 2). The flanking regions and the aadA cassette were purified after electrophoresis of DNA fragments on agarose gels. The fragments were mixed at a 3:1:3 ratio of the upstream flank to the antibiotic resistance cassette to the downstream flank and ligated with T4 DNA ligase. The ligation products were directly used to transform Synechococcus 7002 as described previously (27).
TABLE 1.
Plasmids used in this study
| Plasmids | Relevant characteristics | Phenotypea | Source or Ref. |
|---|---|---|---|
| pSRA2 | Contains a 1.1-kb aadA streptomycin and spectinomycin resistance cassette | ApR SmR SpR | 56 |
| pBluescript II KS(+) | Cloning vector; polylinker | ApR | Agilent Technologies, Inc. (Santa Clara, CA) |
| pRL409 | Contains a 1.2-kb cat chloramphenicol resistance cassette and a 1.5-kb ermC erythromycin resistance cassette | ApR CmR EmR | 57 |
| pLM1 | pBluescript II KS (+) derivative harboring the cat cassette flanked by the 5′- and 3′-flanking regions of desF | ApR CmR | This study |
| pLM2 | pBluescript II KS (+) derivative harboring the ermC cassette flanked by the 3′-flanking regions of chlR and desF | ApR EmR | This study |
| pAQ1Ex-ntR | Contains recombination sites for pAQ1 of Synechococcus 7002, pMB1 ori; PnrtABCD-6803::yfpN-His, aadA between recombination sites | ApR SmR SpR | 28 |
| pAQ1cpcEx | Contains recombination sites for pAQ1 of Synechococcus 7002, pMB1 ori; PcpcBA-6803, aacC1 between recombination sites | ApR GmR | 58 |
| pLM3 | Harbors recombination sites for pAQ1 of Synechococcus 7002, pMB1 ori; PnrtABCD-6803::yfpN-His, aacC1 between recombination sites | ApR GmR | This study |
| pLM4 | PnrtABCD-6803::chlR, aacC1 flanked by recombination sites for pAQ1 of Synechococcus 7002, pMB1 ori | ApR GmR | This study |
| pET-42b | pMB1 ori, lacI, T7lac promoter, aphAII | KmR | Novagen, EMD Millipore |
| pLM5 | pET-42b derivative containing T7lac::chlRN-Strep, pMB1 ori, lacI, kanR | KmR | This study |
| pLM6 | pLM3 derivative with PacsF-II-6803::yfpN-His, aacC1 between pAQ1 recombination sites; pMB1 ori | ApR GmR | This study |
| pCDFDuetTM-1 | CloDF13 ori, lacI, 2 × T7lac promoter, aadA | SmR SpR | Novagen, EMD Millipore, |
| pLM7 | pCDFDuetTM-1 derivative with PacsF-II-6803::yfpN-His, CloDF13 ori, aadA | SmR Sp | This study |
| pAQ1Ex::PcpcBA::yfp | Contains recombination sites for pAQ1 of Synechococcus 7002, pMB1 ori; PcpcBA::yfpN-His, aacC1 between recombination sites | ApR GmR | 37 |
a ApR, ampicillin resistance; CmR, chloramphenicol resistance; EmR, erythromycin resistance; GmR, gentamicin resistance; KmR, kanamycin resistance; SmR, streptomycin resistance; SpR, spectinomycin resistance.
TABLE 2.
Oligonucleotide primers used in this study
| Number | Sequence (relevant restriction sites underlined, 5′–3′ |
|---|---|
| 1 | AAATCTAGATCATCTCGTAAAAGTCAGACCG (XbaI site underlined) |
| 2 | TTTGATATCATTGGCCAAACATACTTCATTG (EcoRV site underlined) |
| 3 | CCGGATATCGCTTAATTTTTTAGGACAATTC (EcoRV site underlined) |
| 4 | TTTCTCGAGAACCGAGTCTTGGTTTAGCGCC (XhoI site underlined) |
| 5 | AAATCTAGAGGCATTCAGGATTTTAATCCC (XbaI site underlined) |
| 6 | TTTGATATCATGAATTCTCAAACAATAGAAC (EcoRV site underlined) |
| 7 | AAAAAGCTTAAATTTGCGTTTGATCATTACC (HindIII site underlined) |
| 8 | TTTCTCGAGGACCACCTCGATCGTCGCCG (XhoI site underlined) |
| 9 | TTTTCTAGAACCGAGTCTTGGTTTAGCGCC (XbaI site underlined) |
| 10 | AAACCATGGCTGACCCCAACCCTTGTAATG (NcoI site underlined) |
| 11 | TTTGGATCCTTAAGCCGATTGCACCGAATC (BamHI site underlined) |
| 12 | TGTTTGGCCACATATGGGATGGAGCCACCCGCAGTTCGAAAAAGGCGCCATGACTGACCCCAACCCTTGTAATG (NdeI site underlined) |
| 13 | AAAAGGATCCTTAAGCCGATTGCACCGAATC (BamHI site underlined) |
| 14 | CCGGAATTCTAAAAACCTCATTGATTTAC (EcoRI site underlined) |
| 15 | ATACCATGGTTAATTAACAGGAGAATC (NcoI site underlined) |
For inactivation of desF, the flanking regions were amplified by PCR using primers 5 and 6 (upstream) and primers 7 and 8 (downstream), respectively. The resulting PCR products were subsequently cloned as XbaI/EcoRV (upstream flanking region) and HindIII/XhoI fragments (downstream flanking region), respectively, into pBluescript II KS(+); finally, the cat gene conferring chloramphenicol resistance was cloned as a 1213-bp EcoRV/HindIII fragment from pRL409 into this plasmid, resulting in pLM1. For deletion of the entire region comprising chlR, acsFII, ho2, hemN, and desF, the flanking regions were amplified using primers 3 and 9 (3′ of chlR) and primers 7 and 8 (3′ of desF). The resulting PCR fragments were cloned as XbaI/EcoRV and HindIII/XhoI fragments, respectively, into pBluescript II KS(+), and ermC conferring erythromycin resistance was cloned as a 1503-bp EcoRV/HindIII fragment from pRL409 into the same plasmid, resulting in pLM2. XhoI-linearized pLM1 and ScaI-linearized pLM2 were used to transform Synechococcus 7002.
To introduce the chlR gene into plasmid pAQ1 of Synechococcus 7002 under control of an inducible promoter, an expression vector having the PnrtABCD promoter of Synechocystis 6803 and aacC1 gene conferring gentamycin resistance was constructed. A 478-bp EcoRI/NdeI fragment comprising the PnrtABCD promoter region of Synechocystis sp. PCC 6803 and a His tag sequence from pAQ1Ex-ntR (28) was cloned into pAQ1cpcEx that had aacC1 as a drug marker, resulting in pLM3. The chlR gene from Synechococcus 7002 was amplified using primers 10 and 11, and the resulting PCR product was cleaved with NcoI and BamHI, resulting in a 199-bp NcoI fragment and a 187-bp NcoI/BamHI fragment. The NcoI/BamHI fragment was first cloned into NcoI/BamHI-digested pLM3 followed by cloning the NcoI fragment into the NcoI-digested pLM3 derivative, finally resulting in pLM4. Both the orientation of the NcoI fragment and the whole PCR-amplified region were confirmed by sequencing. Through NcoI cloning, the His tag-coding sequence of pLM3 was removed, resulting in a non-tagged gene product. However, introduction of the NcoI site at the 5′-end of the chlR gene caused a threonine to alanine exchange of the second amino acid in the resulting protein. The spectinomycin-resistant chlR deletion mutant strain was transformed with ScaI-linearized pLM4.
A Strep-tagged variant of ChlR was constructed for expression and subsequent purification from E. coli. An N-terminal Strep tag-coding sequence and restriction sites were fused to the chlR coding sequence by PCR using primers 12 and 13, and the resulting PCR product was cleaved with NdeI and BamHI, resulting in a 422-bp NdeI/BamHI fragment. This fragment was cloned into NdeI/BamHI-digested pET-42b expression vector, yielding pLM5.
A reporter construct based on the yellow fluorescent protein (YFP) was designed for testing expression levels from the acsFII promoter sequence. To probe expression in Synechococcus 7002, the acsFII promoter region of Synechocystis 6803 was amplified by PCR using primers 14 and 15. The PCR product was digested with EcoRI and NcoI, and the resulting 581-bp EcoRI/NcoI fragment was cloned into EcoRI/NcoI-digested pLM3, replacing the PnrtABCD promoter and yielding pLM6. Finally, ScaI-linearized pLM6 was transformed into wild-type Synechococcus 7002. For expression experiments in E. coli, a 1515-bp EcoRI/XbaI fragment from pLM6, including the Synechocystis 6803 PacsF-II sequence and yfp, was cloned into an EcoRI/XbaI fragment of pCDFDuetTM-1, which only included the origin of replication and the aadA resistance marker, yielding pLM7. For expression experiments in E. coli, plasmids pLM5 and pLM7 were transformed either separately or together into E. coli BL21(DE3). All expression constructions for Synechococcus 7002 and E. coli were verified by DNA sequencing.
RNA Preparation, RNA Sequencing, and Data Analysis
RNAsamples for subsequent cDNA library construction were prepared from frozen cell pellets derived from 25-ml aliquots of liquid culture (pooled from three independent cultures). The RNA preparation and quantitation were performed as described previously (2). Construction of cDNA libraries and SOLiDTM sequencing was performed in the Genomics Core Facility at The Pennsylvania State University (University Park, PA). The cDNA libraries were constructed using the SOLiD Whole Transcriptome Analysis kit (Applied Biosystems) and were barcoded by using the SOLiD Transcriptome Multiplexing kit (Applied Biosystems). The SOLiD ePCR kit and SOLiD Bead Enrichment kit (both Applied Biosystems) were used for processing the samples for sequencing, and the SOLiD 5500 protocol (Applied Biosystems) was used for sequencing. The sequence data have been submitted to the NCBI Sequence Read Archive (SRA) under accession numbers SRX275952 to SRX275958 and SRX268824.
Mapping of cDNA sequences was performed by using the Burrows-Wheeler algorithm (29). The Synechococcus 7002 genome and the sequences for the various drug marker cassettes used were used as the reference genome; four mismatches within the 50-bp reads were allowed (>90% sequence identity). Sequences mapping to ribosomal RNA-coding regions and reads that did not map uniquely were disregarded. The methods for counting the sequences covering each ORF, for calculating the relative transcript abundance (RTA)3 for each ORF, and for comparing RTAs between different data sets were performed as described previously (2). The probability (p values) for equal RTA in the respective comparisons was calculated for each ORF either by using the z-test or the χ2 test as appropriate (for more details, see Ref. 2). The data for all protein-coding ORFs derived from these analyses are listed in supplemental Tables S1 and S2.
Overexpression and Purification of N-terminally Strep-tagged ChlR
For production of N-terminally Strep-tagged ChlR (NStrepChlR), E. coli cells were cultivated in 1 liter of Luria-Bertani medium (10 g liter−1 tryptone, 5 g liter−1 yeast extract, 10 g liter−1 NaCl) containing 30 μg ml−1 kanamycin in 2.8-liter flat bottom flasks at 37 °C with shaking at 120 rpm. When the cultures reached an OD600 nm of 0.6, chlR expression was induced by adding 0.1 mm isopropyl 1-thio-β-d-galactopyranoside, and the culture was further incubated for 4 h. Cells were harvested by centrifugation (10 min, 5000 × g, 4 °C), washed in Buffer A (100 mm Tris/HCl buffer at pH 8.0, 150 mm NaCl), frozen in liquid nitrogen, and stored at −80 °C until required.
Purification of NStrepChlR was performed under oxic conditions. Cells were resuspended in Buffer A at a ratio of 8 ml of buffer/1 g of cells (wet weight), and lysozyme was added to a final concentration of 1 mg ml−1. The cell suspension was stirred at room temperature for 20 min, and cells were disrupted by sonication (Branson Sonifier 450, three intervals at 30 s at level 2.5 and 70%). The sample was kept on ice during sonication. The cell extract was cleared by ultracentrifugation (100,000 × g, 60 min, 4 °C). The resulting soluble extract was loaded on a column containing Strep-Tactin Superflow resin (Iba, Göttingen, Germany) (5-ml bed volume equilibrated with Buffer A). The column was washed with 10 column volumes of Buffer A and eluted with 5 × 3 ml of elution buffer (Buffer A containing 5 mm d-desthiobiotin). Fractions were collected, and protein-containing fractions were pooled and concentrated using a centrifugal ultrafiltration device (Amicon Ultra centrifugal filter, Ultracel 10,000, EMD Millipore, Billerica, MA; 5000 × g). The sample was washed twice using Buffer A and concentrated again to dilute the d-desthiobiotin. The protein concentration was determined by the Bradford method (30) using bovine serum albumin as a standard. The purity of the samples was estimated by visual inspection of SDS-polyacrylamide gels stained by Coomassie Brilliant Blue G-250 (31). Quantitative amino acid analysis of NStrepChlR samples was performed at the Molecular Structure Facility at the University of California-Davis.
Fe-S Cluster Reconstitution and Chemical Determination of Iron
Reconstitution of Fe-S clusters was performed under anoxic conditions as described previously (32). In brief, dithiothreitol (10 mm final concentration), FeCl3 (0.5 mm final concentration), and Na2S (0.8 mm final concentration) were added stepwise to a solution of NStrepChlR (at a final concentration of 0.1 mm in Buffer A). After reconstitution, the sample was concentrated by ultrafiltration (Amicon Pressure Cell, EMD Millipore) with a 10,000-molecular weight cutoff membrane and subjected to ion-exchange chromatography. Molecular sieve chromatography of NStrepChlR was carried out according to a procedure described previously (33) using an ÄKTA (GE Healthcare) liquid chromatography system in a Coy anaerobic chamber. The sample was purified on a HiPrep 16/60 Sephacryl S-200 HR column (GE Healthcare) equilibrated with Buffer A at a flow rate of 0.5 ml min−1. This column was previously calibrated with molecular weight markers as described (34). Protein-containing fractions were pooled and concentrated using a centrifugal ultrafiltration device (Amicon Ultra centrifugal filter, Ultracel 10,000, EMD Millipore). Samples prepared for Mössbauer spectroscopy were reconstituted using 57Fe by the same procedure. Chemical iron determination was performed as described previously (35, 36).
UV-Visible Spectroscopy, Low Temperature EPR, and Mössbauer Spectroscopy
UV-visible spectra were recorded using a modified Cary 14 spectrophotometer (On-Line Instrument Systems, Bogart, GA). Diluted, reconstituted NStrepChlR samples were aliquoted into air-tight quartz cuvettes under anoxic conditions. After spectra of the anoxic samples were recorded, the cuvettes were opened to expose the sample to oxygen. After mixing the sample with air and incubating the sample for 20 min at room temperature, spectra of the oxygen-exposed samples were recorded.
NStrepChlR (300 μl, 57Fe-labeled) that had been reconstituted anoxically for Mössbauer spectroscopy was aliquoted into Mössbauer cups under anoxic conditions, frozen, and stored in liquid nitrogen until measured. A sample of O2-exposed reconstituted NStrepChlR was prepared by exposing the above sample to air, pipetting to mix the solution several times, and then incubating it at ambient atmosphere on ice for 15 min before freezing the sample in liquid nitrogen. Samples for EPR spectroscopy were prepared by diluting the samples 10-fold with Buffer A, placed in calibrated EPR tubes (Wilmad Lab Glass, Vineland, NJ), and frozen anoxically by slow immersion of the tube in liquid nitrogen. EPR samples of reduced NStrepChlR were prepared by addition of 9 μl of 100 mm sodium dithionite in 1 m HEPES buffer, pH 7.5 to a 300-μl sample. After adding dithionite solution, the sample was mixed, incubated for a few minutes on ice, and then frozen as described above.
EPR spectra were acquired on a Bruker ESP300 CW X-Band spectrometer (operating at approximately 9.48 GHz) equipped with a rectangular cavity (TE102) and a continuous flow cryostat (Oxford 910) with a temperature controller (Oxford ITC 503). Spin quantitation was carried out relative to a Cu2+-EDTA standard.
Mössbauer spectra were recorded on spectrometers from WEB Research (Edina, MN). The spectrometer used to acquire the weak field spectra was equipped with a Janis SVT-400 variable temperature cryostat. The spectrometer used to acquire the strong field spectra was equipped with a Janis 8TMOSS-OM-12SVT variable temperature cryostat. The external magnetic field was applied parallel to the γ beam. All isomer shifts quoted are relative to the centroid of the spectrum of α-iron metal at room temperature. Simulation of the Mössbauer spectra was carried out by using the WMOSS spectral analysis software (WEB Research, Edina, MN).
ChlR-dependent YFP Expression in Synechococcus 7002 and in E. coli
Synechococcus 7002 cells harboring the reporter PacsF-II-6803::yfp were grown under the same conditions described above for standard growth conditions or microoxic conditions. Cells harboring a yfp reporter gene on plasmid pAQ3 that was under the control of the very strong PcpcBA promoter (37) was the positive control, and wild-type Synechococcus 7002 cells served as the negative control. Cultures were grown to an OD730 nm between 1.5 and 2.0, and the cell suspension was then adjusted with A+ medium to an OD730 nm of 1.0 for the YFP fluorescence measurements.
For YFP fluorescence measurements in E. coli, BL21(DE3) cells were used that harbored either the expression plasmid for the regulator (pLM5), the YFP reporter plasmid (pLM7), or both plasmids. Cultures were grown in LB medium supplemented with 30 μg kanamycin ml−1 for pLM5 and 50 μg spectinomycin ml−1 for pLM7. An E. coli culture harboring a pAQ1Ex::PcpcBA::yfp derivative with aacC1 as drug marker served as the positive control; 50 μg gentamycin ml−1 was added to the LB medium for these cells. Primary cultures (20 ml of medium in 100-ml Erlenmeyer flasks) were incubated at 37 °C on a shaker at 240 rpm. When these cultures reached an OD600 nm of 0.3, the cultures were split. One-half was transferred into a 10-ml Erlenmeyer flask, which had almost no headspace to produce microoxic conditions. The other half was left in the 100-ml flask to provide strong aeration and thus oxic conditions. For the co-expression experiments in E. coli, expression of NStrepChlR was not additionally induced by addition of isopropyl 1-thio-β-d-galactopyranoside, but expression of the regulator (NStrepChlR) occurred at a basal level due to inherent leakiness of the lacZ promoter system. The cultures were further incubated at 37 °C and 240 rpm shaking for 1.25 and 2.5 h (for oxic and microoxic conditions, respectively), and the cultures were then rapidly chilled on ice to stop further growth and slow down cellular processes (final OD600 nm values were between 1.2 and 1.9). The cells were collected by centrifugation (5 min, 5000 × g, 4 °C), washed in 50 mm Tris-HCl buffer at pH 8.0, and resuspended in the same buffer. Cell suspensions were adjusted with buffer to an OD600 nm of 0.5 for YFP fluorescence measurements.
Fluorescence spectra were taken with an SLM-Aminco 8100C spectrofluorometer, which was modernized for computerized data acquisition by On-Line Instrument Systems. The cell suspensions were excited at 488 nm, and the average of three scans from 500 to 600 nm was calculated.
RESULTS
Deletion of chlR Affects Growth at Lower O2 Levels
To investigate the function of ORF SynPCC7002_A1993, which was annotated as a putative transcriptional regulator, a deletion mutant was constructed by completely replacing the ORF by a drug resistance cassette (aadA) (Fig. 1A). After transformation, the wild-type and mutant alleles segregated completely (Fig. 1B), which indicated that chlR is not an essential gene under the conditions used for selection of transformants and segregation of alleles. Growth of the chlR mutant strain was indistinguishable from that of the wild type when agar plates were incubated in air (Fig. 2A). However, when plates were incubated under an O2-free atmosphere (in a glove box with an atmosphere of CO2:H2:N2, 10:10:80, v/v/v), the chlR mutant failed to grow, and only when traces of oxygen became available from wild-type cells could very small colonies form (Fig. 2B). When cultivated in liquid cultures under microoxic conditions (i.e. vigorous sparging with 1% CO2 and 99% N2 (v/v)), the chlR only grew slightly slower than the wild type (data not shown). Mutants in which desF alone or chlR and the entire acsFII-ho2-hemN-desF operon had been deleted also failed to grow on plates in an anoxic environment (Fig. 2B). These data suggest that DesF, which is likely to replace the essential Δ9-acyl-lipid desaturase DesC (38), may play an essential role in lipid synthesis under microoxic conditions.
FIGURE 1.
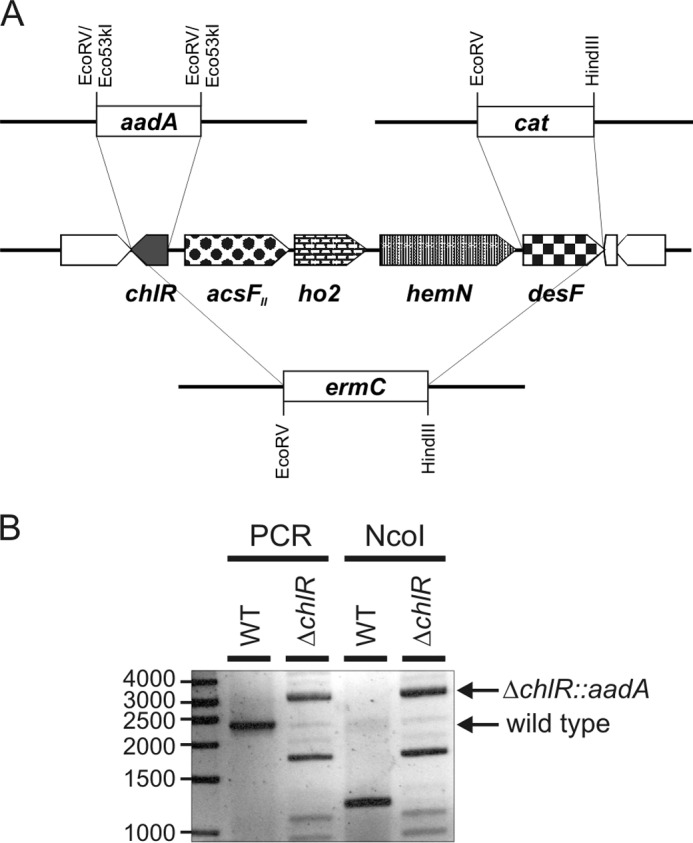
Organization of the acsFII gene cluster and segregation of chlR deletion. A, physical map showing the organization of the acsFII gene cluster and the gene encoding the transcriptional regulator chlR in the genome of Synechococcus 7002. Replacements of chlR, desF, and the entire region encoding chlR to desF by aadA, cat, and ermC cassettes, respectively, by homologous recombination are also illustrated. B, electrophoretic analysis of PCR amplicons produced using DNA from the wild-type strain (WT) or the ΔchlR::aadA deletion mutant of Synechococcus 7002 as template before and after digestion by NcoI. The amplicon produced from a wild-type DNA template was ∼2400 bp, which could be digested into nearly equally sized 1200-bp fragments by NcoI. The ∼3000-bp amplicon produced from the ΔchlR::aadA mutant template had no NcoI sites. The primer pair used to analyze the mutation produced some nonspecific products (that could not be cleaved by NcoI) when the mutant DNA was used as template.
FIGURE 2.

Agar plates showing growth of Synechococcus 7002 WT, chlR mutant (ΔchlR), desF mutant (ΔdesF), and a strain in which the entire region from chlR to desF was deleted (ΔchlR-desF). Cells were streaked on medium A+ plates without antibiotics and grown in air or in an anaerobic chamber in an atmosphere of 10% CO2, 10% H2, and 80% N2. All strains could grow on atmospheric O2 levels, but only the wild type grew under anoxic conditions when the only oxygen available to the cells was that produced by photosynthesis.
Deletion of chlR Abolishes O2-dependent Regulation of the Low O2-induced Operon
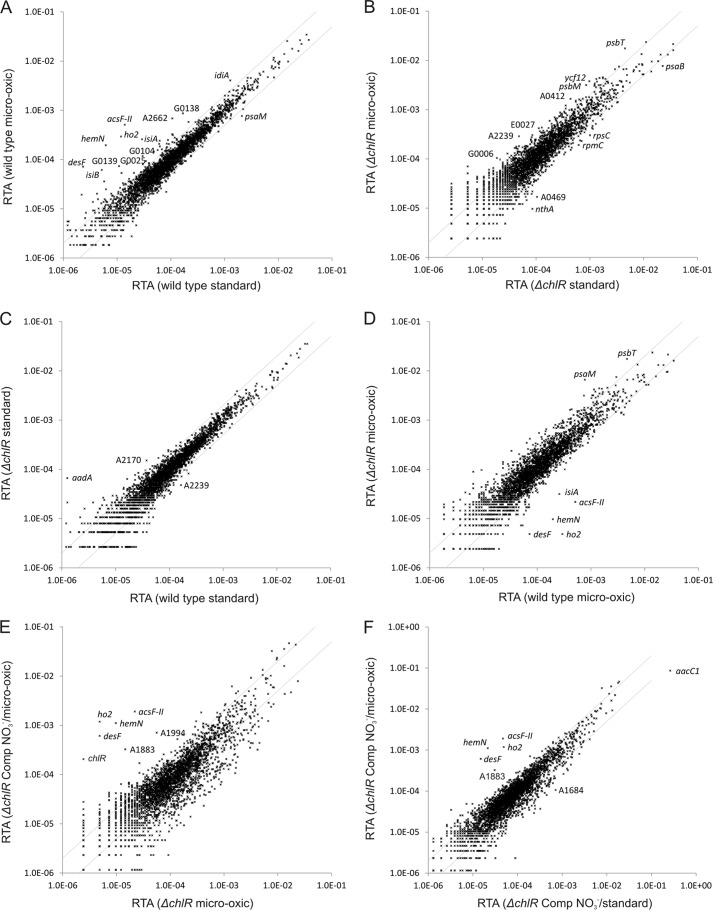
In a previous study on the effects of light, darkness and oxygen on the transcriptome of Synechococcus 7002, we showed that transcript levels of an operon comprising acsFII, ho2, hemN, and desF clearly responded to changing O2 levels. Compared with standard conditions, the relative transcript abundance for these four genes increased when cells were grown under microoxic conditions (2) (see Fig. 3A). Besides these four genes, a few other genes showed increased mRNA levels under microoxic conditions, notably isiA and isiB (Table 3). Global transcriptomes for the chlR mutant strain obtained for cultures grown under the same conditions (microoxic versus standard) were essentially identical (Fig. 3B). Transcript levels for acsFII, ho2, hemN, and desF in cells grown under these two conditions did not differ significantly (p values >0.1; Table 3). The transcriptomes of the chlR mutant and the wild type were identical when cultures were grown under standard conditions (Fig. 3C). However, when the two strains were grown under microoxic conditions, the transcript levels for acsFII, ho2, hemN, and desF were much lower in the chlR mutant compared with the wild type (Fig. 3D and Table 3).
FIGURE 3.
Changes in the RTA of wild-type Synechococcus 7002, ΔchlR mutant, and this mutant complemented with chlR under standard or microoxic conditions. The scatter plots show the RTAs of transcripts from wild-type cells grown under microoxic conditions compared with cells grown under atmospheric O2 (A), the RTAs for cells of the ΔchlR mutant grown under microoxic conditions compared with those for cells grown under atmospheric O2 (B). C, scatter plot showing the RTAs of cells of the ΔchlR strain compared with those for the wild type when both strains were cultivated under atmospheric O2. D, scatter plot showing the RTAs for cells of the ΔchlR mutant compared with those of the wild type when cultures were grown under microoxic conditions. E, scatter plot showing the RTAs of cells for the complemented ΔchlR mutant under derepression conditions (using NO3− as the nitrogen source) to allow expression of chlR compared with the RTA values for the ΔchlR mutant (both strains were grown under microoxic conditions). F, scatter plot showing a comparison of the complemented ΔchlR mutant under derepression conditions (using NO3− as the nitrogen source) when grown under microoxic conditions compared with the same cells grown under atmospheric O2. The values for the wild type under standard conditions (i.e. atmospheric O2 levels) were calculated as the mean of three independent biological replicates. The gray lines show 2-fold changes in either direction. Selected genes are identified by gene locus or abbreviated locus tag number (e.g. A2662 represents SynPCC7002_A2662). Comp, chlR-complemented strain.
TABLE 3.
Ratios of the relative transcript abundance for chlR, acsFII, ho2, hemN, desF, isiA, and isiB
| Gene name | WT microoxic/stda | ΔchlR microoxic/std | ΔchlR/WT std | ΔchlR/WT microoxic | ΔchlR-Comp NO3−/NH4+ std | ΔchlR-Comp NO3−/NH4+ microoxic | ΔchlR-Comp NO3− microoxic/std | ΔchlR-Comp NH4+ microoxic/std | ΔchlR-Comp NO3−/ΔchlR microoxic | ΔchlR-Comp NO3−/WT microoxic |
|---|---|---|---|---|---|---|---|---|---|---|
| chlR | 1.56b | NDb | 0.00 | 0.13 | 26.84 | 8.37 | 0.30 | 0.95b | 85.01 | 10.64 |
| acsFII | 36.82 | 2.05b | 0.77b | 0.04 | 4.14 | 155.44 | 40.89 | 1.09b | 87.74 | 3.75 |
| ho2 | 24.95 | 0.46b | 0.90b | 0.02 | 6.03 | 120.67 | 24.38 | 1.22b | 245.20 | 4.04 |
| hemN | 31.86 | 1.82b | 0.87b | 0.05 | 6.64 | 225.74 | 51.82 | 1.52b | 114.67 | 5.69 |
| desF | 30.86 | 0.61b | 3.42 | 0.07 | 1.85b | 82.89 | 40.96 | 0.91b | 126.32 | 8.51 |
| isiA | 8.88 | 0.74b | 1.46b | 0.12 | 0.25 | 0.50 | 1.79 | 0.91b | 1.59b | 0.19 |
| isiB | 11.93 | 5.47 | 0.51 | 0.24 | 0.40 | 0.47 | 0.99b | 0.85b | 0.80b | 0.19 |
a ΔchlR, chlR mutant; std, standard growth conditions (2); Comp, chlR-complemented strain; ND, not defined due to division by zero.
b p value >0.1.
Controlled Expression of chlR Restores Expression of the acsFII-ho2-hemN-desF Operon
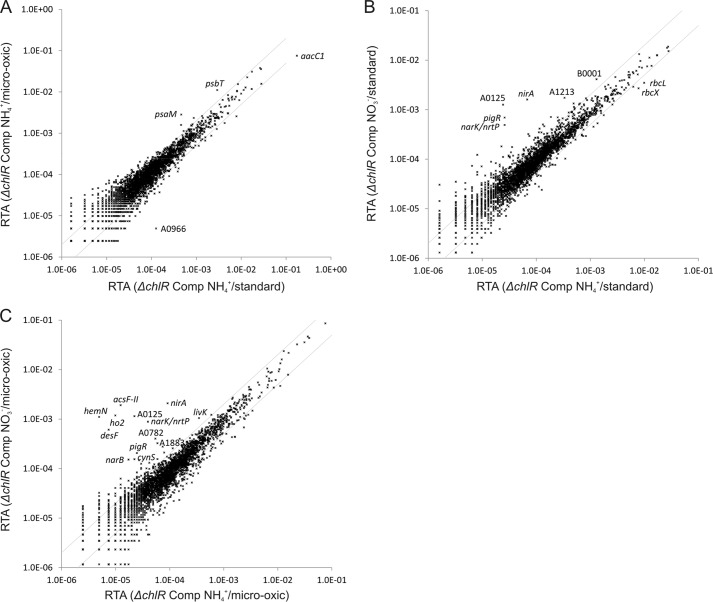
For the expression of ChlR in the chlR deletion mutant strain, the gene was reinserted by taking advantage of the previously described expression system (37) that inserts a gene of interest into pAQ1, which is the smallest plasmid of Synechococcus 7002 and which has the highest copy number of the six plasmids in this strain (37). For expression experiments, the controllable nrtABCD promoter of Synechocystis 6803 that is induced in the absence of ammonia (39) was selected to be able to control the expression levels of chlR. Fig. 3E compares the transcriptomes obtained for permissive conditions for chlR expression (i.e. cells were grown with nitrate as the nitrogen source) with the transcription data obtained for the chlR mutant when both cultures were grown with nitrate under microoxic conditions. Transcript levels for acsFII, ho2, hemN, and desF were 90–250-fold higher in the cells expressing chlR compared with the “background” levels for these transcripts observed in the chlR deletion mutant (Table 3). The much higher transcript levels for the acsFII-ho2-hemN-desF operon in the complemented strain clearly showed that the transcriptional regulation of these genes had been fully restored (Fig. 3E). Notably, transcript levels for isiA and isiB, which were increased in the wild-type strain under microoxic conditions, did not respond to complementation (Table 3).
A comparison of the transcriptome obtained for cells of the complemented chlR expression strain that were grown under microoxic conditions with that of cells grown at an atmospheric O2 level with both cultures grown with nitrate as nitrogen source showed that transcript levels for acsFII, ho2, hemN, and desF were much higher under microoxic conditions, whereas isiA and isiB levels were essentially unchanged (Fig. 3F). This result confirms that regulation of only the acsFII-ho2-hemN-desF operon is highly O2-dependent. When the chlR expression strain was grown with ammonia as the nitrogen source (i.e. chlR expression is repressed), the transcriptomes of cultures grown under microoxic and standard (oxic) conditions were nearly identical (Fig. 4A). When the transcriptome of cells of the chlR expression strain that were grown with nitrate as nitrogen source was compared with the transcriptome of cells grown with ammonia (when both cultures were grown under standard (oxic) conditions), only a few genes had increased transcript levels: besides chlR, these genes encoded the nitrate reductase (narB), the nitrite reductase (nirA), a nitrate/nitrite transporter (nrtP), and SynPCC7002_A0125 (annotated as formate/nitrite transporter) (Fig. 4B). Very high transcript levels for acsFII, ho2, hemN, and desF, however, were observed when the transcriptomes of the chlR expression strain were compared for cells grown on nitrate versus ammonia when the conditions were microoxic (Fig. 4C). In the same comparison, transcript levels for isiA and isiB were slightly lower in nitrate-grown cells compared with ammonia-grown cells, indicating that these genes are not part of the ChlR regulon (Table 3). These experiments show that ChlR is a transcriptional activator that is required only for expression of the acsFII-ho2-hemN-desF operon.
FIGURE 4.
Changes in the RTA of the Synechococcus 7002 ΔchlR deletion mutant complemented with chlR under different O2 levels and on different nitrogen sources. The scatter plots show the RTA values for cells of the ΔchlR mutant complemented with chlR when cells were grown under microoxic conditions relative to atmospheric O2 level (cells were grown with ammonia as the nitrogen source) (A), the RTA values for cells of the ΔchlR mutant complemented with chlR that had been grown under standard conditions with nitrate or ammonia as the nitrogen source (B), and the RTA values for cells of the ΔchlR mutant complemented with chlR when grown under microoxic conditions with nitrate or ammonia as the nitrogen source (C). The gray lines indicate 2-fold changes in transcript level. Selected genes are identified by name or abbreviated locus tag number. Comp, chlR-complemented strain.
The acsFII Promoter from Synechocystis 6803 Interacts with ChlR from Synechococcus 7002
To characterize this gene regulation mechanism further and to assess the compatibility of the oxygen-controlled systems from Synechococcus 7002 and Synechocystis 6803, we fused the acsFII (sll1874) promoter sequence from Synechocystis 6803 to the yfp gene, which encodes the yellow fluorescent protein (37, 40), as a reporter gene. The expression construct was recombined into the endogenous plasmid pAQ1 of the wild-type Synechococcus 7002 (yielding pAQ1::PacsF-II-6803::yfp). The YFP content in cells of Synechococcus 7002 was monitored as YFP-derived fluorescence at 526 nm. In cultures harboring pAQ1::PacsF-II-6803::yfp, relatively strong YFP fluorescence was observed when the cultures were grown under microoxic conditions (Fig. 5). This result clearly shows that ChlR of Synechococcus 7002 recognized the Synechocystis 6803 promoter. The signal intensity reached about 25% of that observed for the positive control having yfp under the control of the strong cpcBA promoter and recombined into the plasmid pAQ3 of Synechococcus 7002 (37). Much weaker YFP fluorescence was also observed from cells that harbored the plasmid pAQ1::PacsF-II-6803::yfp and that were grown under standard growth conditions at atmospheric O2 levels (Fig. 5), but the intensity was about 6-fold lower than that for cells grown under microoxic conditions, which is consistent with the much lower transcript levels observed for the acsFII-ho2-hemN-desF operon under atmospheric oxygen conditions.
FIGURE 5.
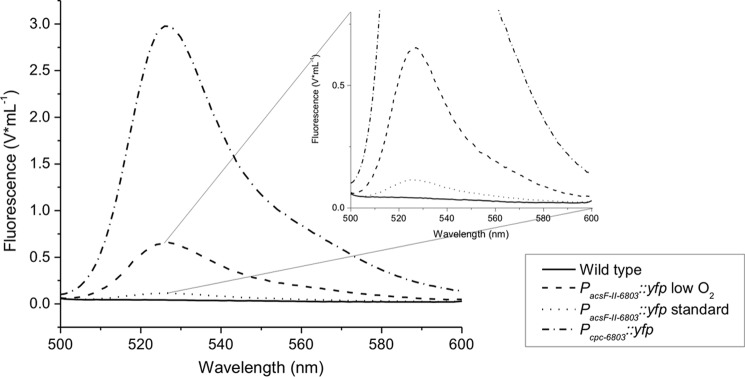
YFP expression in Synechococcus 7002 under control of the acsFII promoter from Synechocystis 6803. YFP expression was detected and quantified as fluorescence for wild-type cells of Synechococcus 7002 (negative control; solid line) and for the YFP expression system under control of the strong PcpcAB promoter from Synechocystis 6803 (positive control; short dash/dotted line) under standard growth conditions. Fluorescence was measured in cultures of the PacsF-II-6803::yfp expression strain that were grown under microoxic conditions (dashed line) and at atmospheric O2 levels (dotted line).
Interaction of ChlR with PacsF-II-6803 Is Independent of Other Cyanobacterium-specific Elements
After demonstrating that ChlR could interact with and regulate the acsFII promoter from Synechocystis 6803, we investigated whether this interaction relies on other cyanobacterium-specific elements. Therefore, chlR (as an N-terminally Strep-tagged derivative) and yfp (under control of PacsF-II-6803) were expressed from separate plasmids (see “Experimental Procedures”) in E. coli BL21(DE3). Maturation of the chromophore of YFP does not occur in the absence of O2 (41). Conveniently, the conditions to allow maturation of the YFP chromophore are incompatible with transcriptional activation, so these two processes can be temporally separated. Chromophore maturation was incomplete in E. coli cultures that were grown under the nearly anoxic conditions used. To achieve full YFP fluorescence signals in E. coli cells, a prolonged incubation (about 2 h) of the cells in a buffer at room temperature and in the presence of O2 was required (data not shown).
E. coli strains with only one of these two components (either chlR or PacsF-II-6803::yfp) did not show any YFP-derived fluorescence, and only the strains carrying both plasmids allowed YFP expression in E. coli (data not shown). The YFP-derived fluorescence signal was intense in cultures grown under microoxic conditions. The maximal fluorescence intensity was about half of that obtained with the very strong cpcBA promoter, which also supports YFP expression in E. coli, for equal cell numbers (Fig. 6). Similar to the situation in Synechococcus 7002, when E. coli cells were grown under oxic conditions with strong aeration, the fluorescence signals were 4–5-fold lower compared with cells grown under microoxic conditions. These results indicate that YFP expression controlled by ChlR relies on the O2 level and interaction between the transcription activator and the promoter. No other components specific to cyanobacteria are apparently required.
FIGURE 6.
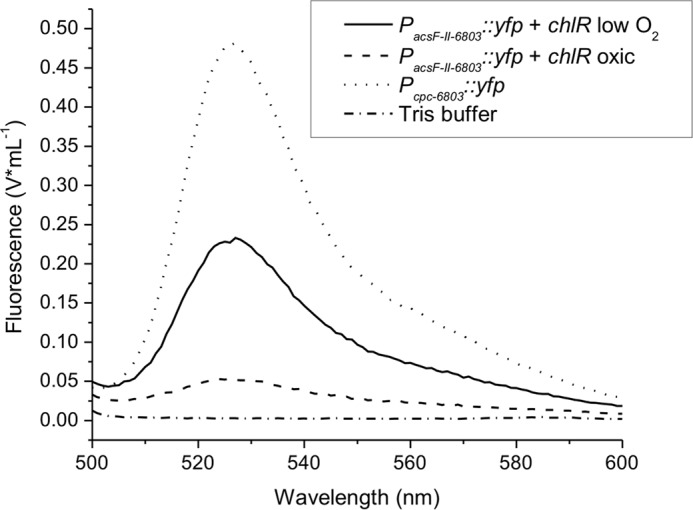
YFP expression in E. coli under control of the acsFII promoter from Synechocystis 6803 and NStrepChlR from Synechococcus 7002. YFP expression was detected and quantified as fluorescence for E. coli BL21(DE3) cells that carried both plasmids pLM7 and pLM5 and that were grown under microoxic conditions or oxic conditions. E. coli cells (strain TOP10F′) with plasmid pAQ1Ex::PcpcBA::yfp grown under oxic conditions served as the positive control. Cells were harvested by centrifugation, washed in 50 mm Tris-HCl buffer at pH 8.0, resuspended in the same buffer, and adjusted to an OD600 nm of 0.5 for the measurements. Cell suspensions were incubated at room temperature and exposed to air; measurements were taken at several times until the YFP fluorescence signal developed fully (data not shown). The fluorescence spectra showing the maximum signal for all condition are as follows: PcpcBA-6803::yfp (pAQ1Ex::PcpcBA::yfp) (positive control; dotted line), PacsF-II-6803::yfp (pLM7) and NStrepChlR (pLM5) microoxic sample (solid line), PacsF-II-6803::yfp and NStrepChlR oxic conditions (dashed line), and Tris-HCl buffer (negative control; dash/dotted line).
ChlR Harbors an Oxygen-labile Fe-S Cofactor
N-terminally Strep-tagged ChlR was heterologously overproduced in E. coli BL21(DE3) and purified to homogeneity under oxic conditions by Strep-Tactin affinity chromatography (Fig. 7A). Preliminary data showed that heterologously produced NStrepChlR contained substoichiometric amounts of iron, and UV-visible spectroscopy revealed a faint absorbance that suggested the presence of an Fe-S cofactor. The small Fe-S cluster yield suggested either that the Fe-S cluster biosynthesis machinery of the host cells could not provide sufficient Fe-S clusters to allow for complete maturation of ChlR or that the purification under oxic conditions led to its complete degradation. By enhancing the iron supply during cultivation and by conducting the purification of NStrepChlR under anoxic conditions, the iron content and the characteristic UV-visible absorbance could be increased to some extent, but ChlR was still mostly devoid of Fe-S clusters.
FIGURE 7.
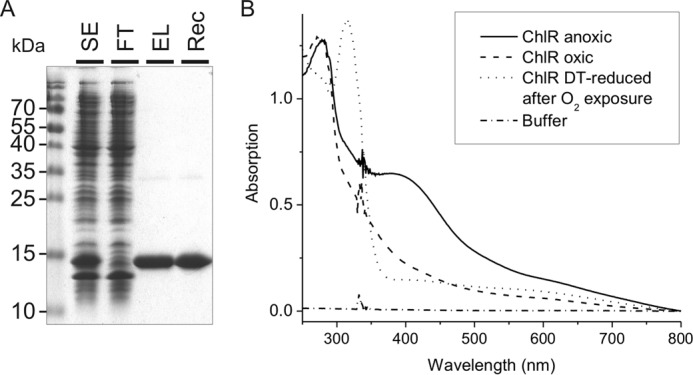
Electrophoretic analysis of fractions from the purification of NStrepChlR and UV-visible spectra of the purified protein. A, proteins from different purification steps of NStrepChlR were separated on a 15% (w/v) SDS-polyacrylamide gel, electrophoresed, and visualized by Coomassie Brilliant Blue staining. SE, soluble extract; FT, flow-through from Strep-Tactin Superflow column (20 μg of protein); EL, eluate after Strep-Tactin Superflow; Rec, purified NStrepChlR protein after reconstitution (5 μg). B, absorption spectra taken for reconstituted NStrepChlR under anoxic conditions, after exposure to air, and after removal of oxygen and reduction with 1 mm sodium dithionite (DT). The measurements were performed in Tris-HCl buffer at pH 8.0 containing 150 mm NaCl (baseline). The protein solution contained 45 μm homodimeric NStrepChlR.
To determine the nature of the metal-containing cofactor, NStrepChlR was next purified under oxic conditions from cells grown oxically, and the protein was then chemically reconstituted under anoxic conditions. After repurification of the protein from the reconstitution solution, the protein had a dark brownish color, and a broad absorption band between 370 and 440 nm was observed in the UV-visible spectrum immediately after reconstitution under an O2-free atmosphere (Fig. 7B). This absorption spectrum is typical of the S→Fe charge transfer bands characteristic of Fe-S proteins. Quantitative amino acid analysis showed that the protein concentration values obtained by the Bradford assay overestimated the protein concentration and that a correction factor of 0.54 was required. An iron analysis for reconstituted NStrepChlR revealed the presence of 4.06 ± 0.07 iron atoms per NStrepChlR homodimer (see below).
When solutions containing reconstituted NStrepChlR were exposed to oxygen (by opening the cuvette, mixing the protein solution with air, and incubating at room temperature for 20 min), the absorbance in the 370–440-nm region of the UV-visible spectrum decreased dramatically (Fig. 7B). Subsequent removal of oxygen and reduction of the same sample with 1 mm sodium dithionite did not restore the original signal but led to a further decrease of the absorbance. These data suggest that the Fe-S cluster in NStrepChlR is irreversibly modified by oxygen.
ChlR Is a Homodimer That Binds One [4Fe-4S] Cluster per Homodimer
To determine the stoichiometry and type of Fe-S cofactors in ChlR, we used a combination of analytical and spectroscopic methods (42). Recombinant apo-NStrepChlR was reconstituted under anoxic conditions, and the resulting protein was chromatographed on a Sephacryl S-200 column to remove excess Fe-S reconstitution reagents and to determine the molecular weight of the protein (Fig. 8). By comparison of the elution time with those for proteins of known molecular weight, the molecular weight of NStrepChlR was determined to be ∼33,000. Considering that the calculated mass of an NStrepChlR monomer is 15,732 Da, this result establishes that reconstituted NStrepChlR is a homodimer in solution.
FIGURE 8.
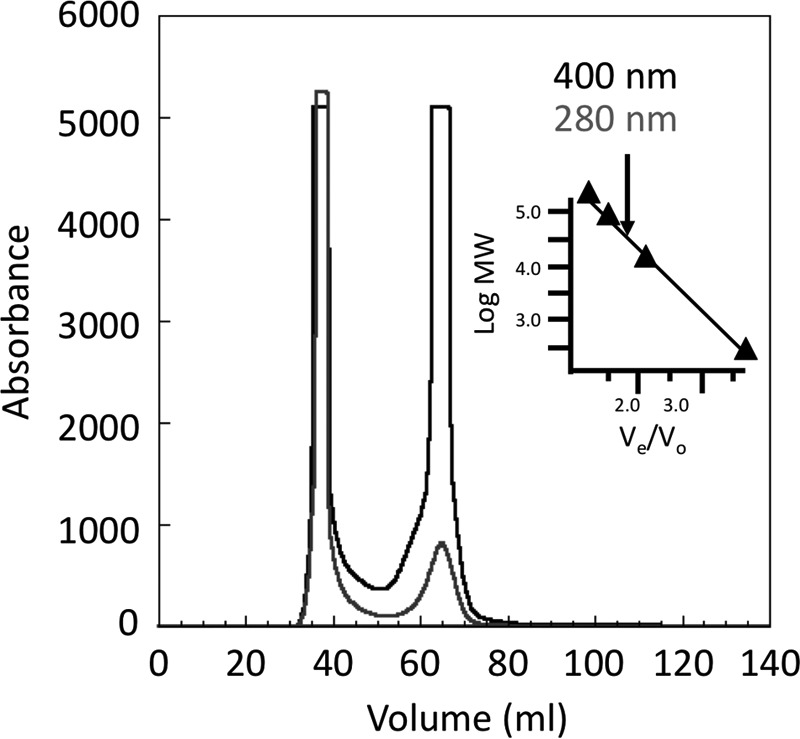
Elution profile for gel exclusion chromatography of reconstituted NStrepChlR on Sephacryl S-200 under anoxic conditions. The black line indicates absorption (relative units) at 400 nm, and the gray line indicates absorption (relative units) at 280 nm. The reconstituted NStrepChlR harboring an Fe-S cluster eluted at 65 ml and excess iron, polysulfides, and protein aggregates eluted at ∼40 ml. The inset shows the standard curve used to determine the molecular weight (MW) of ChlR. Based on the elution time for NStrepChlR (arrow) in comparison with standards, NStrepChlR had a calculated molecular weight of 33,000.
The 4.2-K/53-mT Mössbauer spectrum of NStrepChlR reconstituted with 57Fe under anoxic conditions (Fig. 9A, top, vertical bars) is dominated by a quadrupole doublet with parameters (isomer shift δ = 0.45 mm/s and quadrupole splitting ΔEQ = 1.09 mm/s, 74% of signal intensity; blue line) characteristic of [4Fe-4S]2+ clusters (43, 44). In addition, the pronounced shoulder at ∼0.5 mm/s (green arrow) suggests the presence of a small amount of [2Fe-2S]2+ clusters (quadrupole doublet with δ = 0.28 mm/s and ΔEQ = 0.54 mm/s, ∼15% of signal intensity; green line). The remaining ∼10% of the absorption is broad and featureless (see black arrows) and could emanate either from Fe-S clusters with half-integer spin ground state or from unspecifically bound iron. The EPR spectrum of NStrepChlR reconstituted under anoxic conditions (Fig. 9B, red line) demonstrated largely the absence of Fe-S clusters with half-integer spin ground state. The weak signal with gav = 2.006 could emanate from a small amount (∼0.01 eq) of [3Fe-4S]+ cluster, but this small amount is beyond the detection limit of Mössbauer spectroscopy. Therefore, the broad features in the Mössbauer spectrum are assigned to unspecifically bound iron. The 4.2-K/6-T Mössbauer spectrum (Fig. 9A, bottom) confirms the presence of [4Fe-4S]2+ and [2Fe-2S]2+ clusters, which have diamagnetic ground states. The 6-T spectrum can be simulated with the parameters obtained from the 53-mT spectrum: asymmetry parameters η = 0.8 ([4Fe-4S]2+ cluster; blue line) and η = 0 ([2Fe-2S]2+ cluster; green line), respectively, and assuming an S = 0 ground state for both. Together with the iron and protein analyses, these results suggest the presence of 0.74 eq of [4Fe-4S]2+ clusters and 0.30 eq of [2Fe-2S]2+ clusters per NStrepChlR dimer.
FIGURE 9.

Mössbauer and EPR spectra of reconstituted NStrepChlR transcription activator. A, Mössbauer spectra of 57Fe-labeled reconstituted NStrepChlR (630 μm homodimeric NStrepChlR). The spectra were recorded at 4.2 K in magnetic fields of 53 mT (top, vertical bars) and 6 T (bottom, vertical bars) applied parallel to the γ beam. Simulations with parameters quoted in the text corresponding to the [4Fe-4S]2+ and [2Fe-2S]2+ clusters are shown in blue and green, respectively, whereas the black line corresponds to their summation. The green and black arrows indicate typical features associated with [2Fe-2S]2+ and adventitiously bound iron. B, continuous wave EPR spectra of anoxically reconstituted NStrepChlR (red line), anoxically reconstituted NStrepChlR after reduction with 5 mm sodium dithionite (DT) (blue line), and anoxically reconstituted NStrepChlR exposed to O2 for 30 min (black trace). Experimental conditions were as follows: microwave power, 0.63 milliwatt; modulation amplitude, 0.5 mT; conversion time, 0.082 s; temperature, 10 K; microwave frequency, 9.480 GHz; sample concentration, 63 μm dimeric NStrepChlR.
The EPR spectrum of dithionite-reduced, reconstituted NStrepChlR (Fig. 9B, blue line) exhibits a nearly axial spectrum with principal g values of gx = 2.04, gy = 1.92, and gz = 1.89 and relaxation properties characteristic of those of [4Fe-4S]+ clusters (45). Spin quantitation using a Cu2+ standard yielded 0.77 spin per NStrepChlR homodimer, suggesting that the [4Fe-4S]2+ cluster is (nearly) quantitatively reduced.
Exposure of NStrepChlR reconstituted anoxically to O2 was monitored by Mössbauer and EPR spectroscopies. The Mössbauer spectra recorded before and after O2 exposure (Fig. 10, top and middle, respectively) reveal pronounced changes. The progress of the reaction can be demonstrated from the difference spectrum (Fig. 10, bottom) in which the features pointing downward are converted to those pointing upward upon O2 exposure. Analysis of the difference spectrum reveals that the [4Fe-4S]2+ clusters (50%; 0.50 eq of [4Fe-4S]2+ per dimer; blue line) are converted to [2Fe-2S]2+ clusters (25% of total iron; 0.50 eq [2Fe-2S]2+ per dimer; green line). The missing intensity can be identified by comparison of the experimental difference spectrum with the superposition of the two quadrupole doublets (red line). The line at ∼+3 mm/s occurs at a position typical of octahedrally nitrogen/oxygen-coordinated high spin Fe(II) (∼10%; based on the intensity of this line). In addition, there is a broad, featureless absorption pointing upward. This fraction is assigned to nonspecifically bound iron because the EPR spectrum of reconstituted NStrepChlR after exposure to O2 exhibits only weak features attributable to [3Fe-4S]+ clusters (Fig. 9B, black line). The EPR signal with gav ≈ 2.02 amounts after integration to 0.04 spin per NStrepChlR dimer.
FIGURE 10.

Mössbauer spectra of the O2-exposed reconstituted NStrepChlR transcription regulator. Mössbauer spectra recorded for 57Fe-labeled, anoxically reconstituted NStrepChlR (630 μm homodimeric NStrepChlR) (top) and after exposure of the anoxic sample (middle) for 15 min to atmospheric oxygen are shown. The spectra were recorded at a temperature of 4.2 K and in a magnetic field of 53 mT applied parallel to the γ beam. On the anoxic-oxic difference spectrum (bottom, vertical bars), the simulations corresponding to the [4Fe-4S]2+ and [2Fe-2S]2+ clusters are shown in blue and green, respectively, whereas the red trace corresponds to their summation. The arrow indicates the high energy line of the high-spin Fe(II) formed upon O2 exposure.
Taken together, the spectroscopic and analytical methods reveal that NStrepChlR reconstituted under anoxic conditions harbors 0.74 [4Fe-4S]2+, 0.30 [2Fe-2S]2+, and 0.01 [3Fe-4S]+ clusters per dimer. Upon exposure to O2 for 15 min, the majority of [4Fe-4S]2+ (0.50 eq) are converted to [2Fe-2S]2+ (0.50 eq), whereas the remaining two irons of the disassembled cluster are presumably released into solution because they give rise to Mössbauer features associated with high spin Fe(II) in solution and an aggregated, nonspecific form of iron.
DISCUSSION
In this study, we showed that the product of ORF SYNPCC7002_A1993, annotated as a putative transcriptional regulator, is an oxygen-sensitive transcriptional activator for an operon of four genes, acsFII, ho2, hemN, and desF. Three of the resulting enzymes catalyze important reactions of photosynthetic pigment biosynthesis under microoxic conditions. HemN acts in heme biosynthesis and converts coproporphyrinogen III to protoporphyrinogen IX, AcsFII (ChlAII) converts Mg-protoporphyrin IX monomethyl ester into 3,8-divinylprotochlorophyllide in chlorophyll a biosynthesis, and Ho2 oxidatively cleaves the tetrapyrrole ring of heme to produce biliverdin (7, 15, 16, 23). Under atmospheric O2 levels, these same reactions are catalyzed by HemF, AcsFI (ChlAI), and Ho1 (Hox), respectively. Although the exact function of desF has not yet been demonstrated, based on sequence similarity, it is likely that this protein substitutes for the essential fatty acid desaturase DesC under microoxic conditions (46). The desF gene is often absent from similar operons in other cyanobacteria (e.g. Synechocystis 6803). The paralogous enzymes encoded in such operons, which are presumably optimized to function under microoxic conditions, occur in numerous cyanobacteria. Similar gene clusters like the acsFII-ho2-hemN-desF cluster of Synechococcus 7002 are present in many cyanobacterial strains from different habitats (data not shown); however, the gene composition of these clusters is variable. For example, Leptolyngbya sp. strain PCC 7376 has a similar five-gene operon that includes a psbA paralog.
A very similar MarR-type transcriptional regulator (sll1512) has recently been reported in Synechocystis 6803 (25). That study demonstrated that a constitutively activated variant of ChlR binds to the promoter sequences of acsFII (chlAII) and psbAI. Transcript levels of these genes and of hemN and ho2, the other two genes of the acsFII (chlAII) operon, are expressed at higher levels under microoxic conditions (24). In Synechococcus 7002, however, no changes of the transcript levels of photosystem-related genes and in particular of the three paralogs of psbA were observed in response to microoxic or anoxic conditions (2). Changes of the transcript level of a few other genes related to iron uptake or stress, isiA and isiB in particular, were observed in some microoxic samples (Table 3). This appears to be a secondary effect due to differences in iron availability under low O2 levels because our experimental setup did not allow rigorous control of the O2 level in liquid cultures. The results of our complementation experiments, however, showed clearly that isiA and isiB are not regulated by ChlR (Table 3). Only the four genes of the acsFII-ho2-hemN-desF operon specifically responded to changing O2 levels.
MarR-type transcription regulators typically form homodimers (47), and it was proposed that ChlR of Synechocystis 6803 similarly forms a homodimer that binds to the acsFII (chlAII) promoter region when activated (25). In this study, we show experimentally that ChlR from Synechococcus 7002 forms homodimers in vitro and can activate transcription from the acsFII (chlAII) promoter of Synechocystis 6803 in E. coli in vivo and that no other cyanobacterium-specific factors are required for O2 sensing and transcription activation. By placing a yfp reporter gene under the control of the inducible acsFII (chlAII) promoter and by transferring the system into both Synechococcus 7002 and E. coli, we demonstrated that this system can be used to express genes in a manner that is solely controllable by the oxygen level. This adds to a growing list of expression systems for cyanobacteria, and this system should be especially useful for controlling the expression of hydrogenase genes in cells that are fermenting or in cells that at least have minimal intracellular O2 levels.
MarR-type regulators comprise a broad family of transcription activators and repressors that regulate a variety of stress responses and other cellular processes, and specific binding of ligands has been shown for some examples (47). The purified, reconstituted MarR-type transcription activator ChlR of Synechococcus 7002 harbors an Fe-S cluster as an oxygen-sensitive prosthetic group. A combination of spectroscopic and analytical methods revealed the presence of 0.74 [4Fe-4S]2+ clusters and 0.30 [2Fe-2S]2+ clusters, i.e. a total of 1.04 clusters per ChlR homodimer. The presence of some [2Fe-2S] clusters and a minute amount of [3Fe-4S]+ clusters in ChlR that had been reconstituted and purified under anoxic conditions was presumably due to minor O2 contamination during the purification process and manipulations required to prepare the protein for spectroscopic measurements. Our findings together with the previously described homodimeric structure of other MarR-type regulators as well as the ChlR homolog of Synechocystis 6803 (ChlR) (25, 47) strongly suggest that one [4Fe-4S] cluster is bound per ChlR homodimer. Database searches show that ChlR-type regulators occur in numerous cyanobacteria derived from a wide variety of habitats, but ChlR homologs do not seem to occur in all cyanobacteria. MarR family members also occur in cyanobacteria that do not have acsFII-ho2-hemN-desF operons, but it is presently unknown what these other transcription factors control.
If one considers all cyanobacterial MarR family members, only one Cys residue (the second cysteine; residue 14 in ChlR from Synechococcus 7002) is conserved (see Fig. 11). This led Aoki et al. (25) to suggest that this cysteine residue might form a sulfenic acid or S-thiolated derivative under oxic conditions (non-activating ChlR) that could be reduced to a free thiol under microoxic conditions (activating ChlR) (25). However, if one only considers those MarR family members that are most similar to ChlR (also restricting the analysis to organisms that have operons similar to acsFII-ho2-hemN-desF), a different pattern emerges (Fig. 11). Comparison of this more limited set of ChlR homologs shows that these proteins have four conserved Cys residues; two occur near the N terminus, and two occur near the C terminus. The C-terminal pair of Cys residues always occurs in the pattern Cys-X-Cys, whereas the N-terminal pair of Cys residues occurs in the motif Cys-X4–6-Cys-Pro. The N-terminal Cys motif is reminiscent of a portion of the Cys motif for [4Fe-4S] ferredoxins (i.e. Cys-X2-Cys-X2-Cys-X3-Cys-Pro), and furthermore, these Cys residues occur in close proximity to the N-terminal dimerization domain for MarR proteins. The C-terminal pair of Cys residues occurs in a motif that might bind a metal ion or form a disulfide bond that could respond via thioredoxin to intracellular redox conditions. Because ChlR does not contain a Cys motif typical for Fe-S cluster ligation, we propose that the [4Fe-4S] cluster is bound at the dimer interface by two N-terminal Cys motifs. As shown here, it is very likely that the [4Fe-4S] cluster is the O2-sensing component. The role of the Cys residues of Synechococcus 7002 ChlR as ligands to the [4Fe-4S] cluster is presently unresolved and must be clarified by further studies.
FIGURE 11.
Sequence comparison of ChlR homologs from diverse cyanobacteria. Cys residues are shaded in gray. The shaded aspartic acid residue in the Synechococcus 6803 sequence causes constitutive activation of ChlR when mutated to His (25). Hyphens denote insertions/deletions introduced to optimize the alignment. In the consensus line, stars indicate absolutely conserved residues, and colons and periods indicate conservative replacements. Oscil_6506, Oscillatoria sp. PCC 6506; Trichod_IMS101, Trichodesmium erythraeum strain IMS101; Arthros_NIES-39, Arthrospira platensis strain NIES-39; Arthros_8005, Arthrospira sp. PCC 8005; Leptol_6406, Leptolyngbya sp. PCC 6406; Leptol_HeronIsJ, Leptolyngbya sp. strain Heron Island J; Leptol_7376, Leptolyngbya sp. strain PCC 7376; Syn_6803, Synechocystis sp. strain PCC 6803; Syn_7002, Synechococcus sp. strain PCC 7002; Oscil_7112, Oscillatoria sp. strain PCC 7112; Psanab_6802, Pseudanabaena sp. strain PCC 6802; L_boryana, Leptolyngbya boryana; Acaryoc_MBIC11017, Acaryochloris sp. MBIC11017; Acaryoc_CCMEE5410, Acaryochloris sp. strain CCMEE 5410.
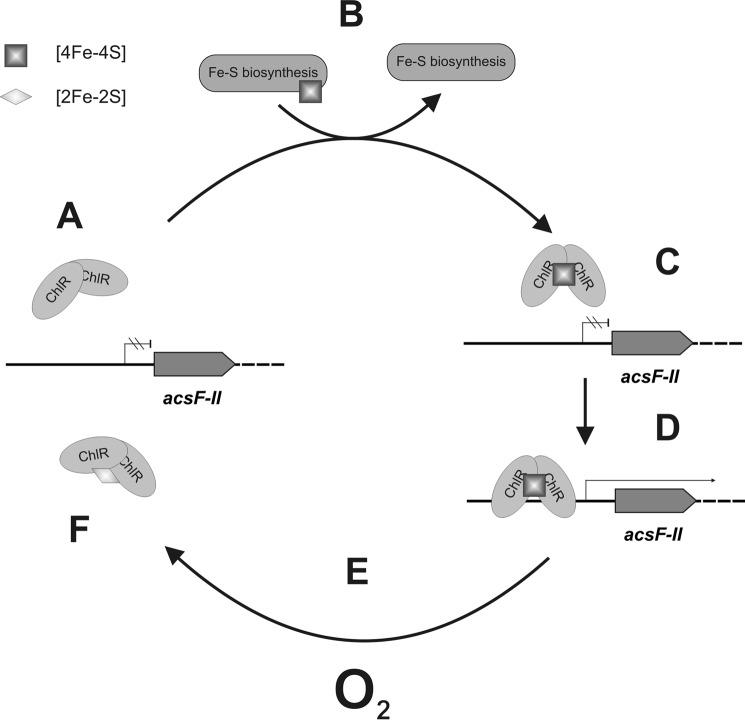
Upon exposure to O2, the [4Fe-4S]2+ cluster of ChlR was converted to a [2Fe-2S]2+ cluster. The remaining two irons of the [4Fe-4S] cluster are apparently released into solution because they exhibit Mössbauer features typical of high-spin Fe(II) in solution and aggregated, nonspecific iron. This degradation mechanism is strikingly similar to the O2-mediated conversion of the [4Fe-4S]2+ cluster of the FNR transcription factor into a [2Fe-2S]2+ cluster (48–50), which is known to be uniquely coordinated by cysteine persulfide ligands rather than cysteinate residues (51). Based on the homodimeric structure of ChlR and the observed structural changes of the Fe-S cofactor, we propose a plausible and testable model for the O2-dependent transcriptional regulation of the acsFII-ho2-hemN-desF operon (Fig. 12). Under oxic conditions, ChlR exists as a cofactor-free precursor (A), which cannot activate transcription from the acsFII promoter. The cellular Fe-S cluster biosynthesis machinery (52) inserts a [4Fe-4S] cluster into homodimeric ChlR (B). When the O2 level is sufficiently low (microoxic conditions), the [4Fe-4S] cluster is stable (C). This conformation of the regulator allows ChlR to bind to the acsFII promoter and activate transcription of the acsFII-ho2-hemN-desF operon (D). When the regulator is exposed to oxygen (E), the [4Fe-4S]2+ cluster is degraded to a [2Fe-2S]2+ cluster (or probably beyond), resulting in a conformational change of the homodimer (F) and causing its dissociation from the acsFII promoter sequence. As a consequence, transcription of the acsFII-ho2-hemN-desF operon ceases. The fate of ChlR containing a [2Fe-2S]2+ cluster is presently unknown. It is possible that ChlR is proteolytically degraded, or alternatively, the [4Fe-4S] cluster can be restored either by disassembly of the [2Fe-2S] cluster followed by reinsertion by the Fe-S cluster biosynthesis machinery or by direct regeneration of the [2Fe-2S] cluster as was observed for the FNR transcription factor (51). Ongoing experiments may establish the maintenance pathway of ChlR.
FIGURE 12.
Model for O2-dependent regulation by ChlR. A homodimer of ChlR lacking Fe-S clusters (A) cannot bind to the acsFII promoter. After insertion of a [4Fe-4S] cluster (B) by the Fe-S cluster biogenesis machinery, ChlR can bind to its operator near the acsFII promoter (C) and activate transcription (D). Exposure to oxygen (E) converts the [4Fe-4S] cluster to a [2Fe-2S] cluster, and the transcription activator loses its ability to bind to the acsFII promoter (F). It is presently not clear whether an Fe-S cluster can be restored or reinserted into the inactivated protein or whether this protein turns over and must be resynthesized.
SufR is another cyanobacterial transcription factor described that utilizes [4Fe-4S] clusters as prosthetic groups (53–55). SufR is a member of the ArsR superfamily and is a transcriptional repressor of the suf regulon in cyanobacteria (53). SufR forms homodimers that ligate two oxygen-labile [4Fe-4S] clusters, which occur in a mixture of S = 1/2 and 3/2 ground spin states (54). SufR appears to utilize at least one non-cysteine ligand to each [4Fe-4S] cluster. Damage to the Fe-S clusters and/or a change in the occupancy of the [4Fe-4S] clusters in SufR, which could result from redox stress, oxidative stress, or iron limitation, presumably causes a conformational change that leads to derepression of the suf regulon (54). SufR additionally regulates its own expression.
In conclusion, this study on ChlR not only provides insight into a very specific cyanobacterial, O2-dependent regulation mechanism but also provides a solely O2-responsive expression system for O2-sensitive enzymes. This system will be particularly interesting for expression of hydrogenases or other enzymes involved in fermentative pathways.
Supplementary Material
Acknowledgments
We gratefully acknowledge Simon Yeung and Dr. Weibing Dong for assistance in making some initial EPR experiments with reconstituted ChlR and for helpful discussions. We also thank Dr. Squire Booker for the use of the gel filtration column and Dr. Tyler Grove for technical assistance in performing the chromatography.
This work was supported by Air Force Office of Scientific Research Multidisciplinary University Research Initiative Grant FA9550-11-1-0148 (to D. A. B.) and National Science Foundation Grant MCB-1021725 (to D. A. B. and J. H. G.).

This article contains supplemental Tables S1 and S2.
- RTA
- relative transcript abundance
- NStrepChlR
- N-terminally Strep-tagged ChlR
- T
- teslas
- mT
- milliteslas.
REFERENCES
- 1. Bryant D. A. (1994) The Molecular Biology of Cyanobacteria, Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- 2. Ludwig M., Bryant D. A. (2011) Transcription profiling of the model cyanobacterium Synechococcus sp. strain PCC 7002 by NextGen (SOLiDTM) sequencing of cDNA. Front. Microbiol. 2, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ludwig M., Bryant D. A. (2012b) Synechococcus sp. strain PCC 7002 transcriptome: acclimation to temperature, salinity, oxidative stress, and mixotrophic growth conditions. Front. Microbiol. 3, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ludwig M., Bryant D. A. (2012a) Acclimation of the global transcriptome of the cyanobacterium Synechococcus sp. strain PCC 7002 to nutrient limitations and different nitrogen sources. Front. Microbiol. 3, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cannon W. R., Rawlins M. M., Baxter D. J., Callister S. J., Lipton M. S., Bryant D. A. (2011) Large improvements in MS/MS-based peptide identification rates using a hybrid analysis. J. Proteome Res. 10, 2306–2317 [DOI] [PubMed] [Google Scholar]
- 6. Bennette N. B., Eng J. F., Dismukes G. C. (2011) An LC-MS-based chemical and analytical method for targeted metabolite quantification in the model cyanobacterium Synechococcus sp. PCC 7002. Anal. Chem. 83, 3808–3816 [DOI] [PubMed] [Google Scholar]
- 7. Minamizaki K., Mizoguchi T., Goto T., Tamiaki H., Fujita Y. (2008) Identification of two homologous genes, chlAI and chlAII, that are differentially involved in isocyclic ring formation of chlorophyll a in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 283, 2684–2692 [DOI] [PubMed] [Google Scholar]
- 8. Porra R. J., Schäfer W., Gad'on N., Katheder I., Drews G., Scheer H. (1996) Origin of the two carbonyl oxygens of bacteriochlorophyll a. Eur. J. Biochem. 239, 85–92 [DOI] [PubMed] [Google Scholar]
- 9. Chew A. G., Bryant D. A. (2007) Chlorophyll biosynthesis in bacteria: the origins of structural and functional diversity. Annu. Rev. Microbiol. 61, 113–129 [DOI] [PubMed] [Google Scholar]
- 10. Bryant D. A., Liu Z. (2013) in Genome Evolution of Photosynthetic Bacteria (Beatty J. T., ed) pp. 99–150, Elsevier, New York [Google Scholar]
- 11. Ouchane S., Steunou A.-S., Picaud M., Astier C. (2004) Aerobic and anaerobic Mg-protoporphyrin monomethyl ester cyclases in purple bacteria: a strategy adopted to bypass the repressive oxygen control system. J. Biol. Chem. 279, 6385–6394 [DOI] [PubMed] [Google Scholar]
- 12. Neilson J. A., Durnford D. G. (2010) Structural and functional diversification of the light-harvesting complexes in photosynthetic eukaryotes. Photosynth. Res. 106, 57–71 [DOI] [PubMed] [Google Scholar]
- 13. Sidler W. A. (1994) in The Molecular Biology of Cyanobacteria (Bryant D. A., ed) pp. 139–216, Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- 14. Beale S. I. (1993) Biosynthesis of phycobilins. Chem. Rev. 93, 785–802 [Google Scholar]
- 15. Zhang X., Migita C. T., Sato M., Sasahara M., Yoshida T. (2005) Protein expressed by the ho2 gene of the cyanobacterium Synechocystis sp. PCC 6803 is a true heme oxygenase. Properties of the heme and enzyme complex. FEBS J. 272, 1012–1022 [DOI] [PubMed] [Google Scholar]
- 16. Aoki R., Goto T., Fujita Y. (2011) A heme oxygenase isoform is essential for aerobic growth in the cyanobacterium Synechocystis sp. PCC 6803: modes of differential operation of two isoforms/enzymes to adapt to low oxygen environments in cyanobacteria. Plant Cell Physiol. 52, 1744–1756 [DOI] [PubMed] [Google Scholar]
- 17. Yilmaz M., Kang I., Beale S. I. (2010) Heme oxygenase 2 of the cyanobacterium Synechocystis sp. PCC 6803 is induced under a microaerobic atmosphere and is required for microaerobic growth at high light intensity. Photosynth. Res. 103, 47–59 [DOI] [PubMed] [Google Scholar]
- 18. Layer G., Heinz D. W., Jahn D., Schubert W.-D. (2004) Structure and function of radical SAM enzymes. Curr. Opin. Chem. Biol. 8, 468–476 [DOI] [PubMed] [Google Scholar]
- 19. Troup B., Hungerer C., Jahn D. (1995) Cloning and characterization of the Escherichia coli hemN gene encoding the oxygen-independent coproporphyrinogen III oxidase. J. Bacteriol. 177, 3326–3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Layer G., Moser J., Heinz D. W., Jahn D., Schubert W.-D. (2003) Crystal structure of coproporphyrinogen III oxidase reveals cofactor geometry of radical SAM enzymes. EMBO J. 22, 6214–6224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Troup B., Jahn M., Hungerer C., Jahn D. (1994) Isolation of the hemF operon containing the gene for the Escherichia coli aerobic coproporphyrinogen III oxidase by in vivo complementation of a yeast HEM13 mutant. J. Bacteriol. 176, 673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phillips J. D., Whitby F. G., Warby C. A., Labbe P., Yang C., Pflugrath J. W., Ferrara J. D., Robinson H., Kushner J. P., Hill C. P. (2004) Crystal structure of the oxygen-dependant coproporphyrinogen oxidase (Hem13p) of Saccharomyces cerevisiae. J. Biol. Chem. 279, 38960–38968 [DOI] [PubMed] [Google Scholar]
- 23. Goto T., Aoki R., Minamizaki K., Fujita Y. (2010) Functional differentiation of two analogous coproporphyrinogen III oxidases for heme and chlorophyll biosynthesis pathways in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 51, 650–663 [DOI] [PubMed] [Google Scholar]
- 24. Summerfield T. C., Toepel J., Sherman L. A. (2008) Low-oxygen induction of normally cryptic psbA genes in cyanobacteria. Biochemistry 47, 12939–12941 [DOI] [PubMed] [Google Scholar]
- 25. Aoki R., Takeda T., Omata T., Ihara K., Fujita Y. (2012) MarR-type transcriptional regulator ChlR activates expression of tetrapyrrole biosynthesis genes in response to low-oxygen conditions in cyanobacteria. J. Biol. Chem. 287, 13500–13507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stevens S. E., Porter R. D. (1980) Transformation in Agmenellum quadruplicatum. Proc. Natl. Acad. Sci. U.S.A. 77, 6052–6056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frigaard N. U., Sakuragi Y., Bryant D. A. (2004b) Gene inactivation in the cyanobacterium Synechococcus sp. PCC 7002 and the green sulfur bacterium Chlorobium tepidum using in vitro-made DNA constructs and natural transformation. Methods Mol. Biol. 274, 325–340 [DOI] [PubMed] [Google Scholar]
- 28. Xu Y. (2010) Synechococcus sp. PCC 7002: a Robust and Versatile Cyanobacterial Platform for Biofuels Development. Ph.D. thesis, The Pennsylvania State University [Google Scholar]
- 29. Li H., Durbin R. (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 31. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 32. Saunders A. H., Griffiths A. E., Lee K.-H., Cicchillo R. M., Tu L., Stromberg J. A., Krebs C., Booker S. J. (2008) Characterization of quinolinate synthases from Escherichia coli, Mycobacterium tuberculosis, and Pyrococcus horikoshii indicates that [4Fe-4S] clusters are common cofactors throughout this class of enzymes. Biochemistry 47, 10999–11012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cicchillo R. M., Baker M. A., Schnitzer E. J., Newman E. B., Krebs C., Booker S. J. (2004) Escherichia coli l-serine deaminase requires a [4Fe-4S] cluster in catalysis. J. Biol. Chem. 279, 32418–32425 [DOI] [PubMed] [Google Scholar]
- 34. Grove T. L., Ahlum J. H., Qin R. M., Lanz N. D., Radle M. I., Krebs C., Booker S. J. (2013) Further characterization of Cys-type and Ser-type anaerobic sulfatase maturating enzymes suggests a commonality in the mechanism of catalysis. Biochemistry 52, 2874–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beinert H. (1978) Micro methods for the quantitative determination of iron and copper in biological material. Methods Enzymol. 54, 435–445 [DOI] [PubMed] [Google Scholar]
- 36. Kennedy M. C., Kent T. A., Emptage M., Merkle H., Beinert H., Münck E. (1984) Evidence for the formation of a linear [3Fe-4S] cluster in partially unfolded aconitase. J. Biol. Chem. 259, 14463–14471 [PubMed] [Google Scholar]
- 37. Xu Y., Alvey R. M., Byrne P. O., Graham J. E., Shen G., Bryant D. A. (2011) Expression of genes in cyanobacteria: adaptation of endogenous plasmids as platforms for high-level gene expression in Synechococcus sp. PCC 7002. Methods Mol. Biol. 684, 273–293 [DOI] [PubMed] [Google Scholar]
- 38. Sakamoto T., Bryant D. A. (1997) Temperature-regulated mRNA accumulation and stabilization for fatty acid desaturase genes in the cyanobacterium Synechococcus sp. strain PCC 7002. Mol. Microbiol. 23, 1281–1292 [DOI] [PubMed] [Google Scholar]
- 39. Flores E., Frías J. E., Rubio L. M., Herrero A. (2005) Photosynthetic nitrate assimilation in cyanobacteria. Photosynth. Res. 83, 117–133 [DOI] [PubMed] [Google Scholar]
- 40. Cormack B. P., Valdivia R. H., Falkow S. (1996) FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173, 33–38 [DOI] [PubMed] [Google Scholar]
- 41. Heim R., Prasher D. C., Tsien R. Y. (1994) Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc. Natl. Acad. Sci. U.S.A. 91, 12501–12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lanz N. D., Grove T. L., Gogonea C. B., Lee K. H., Krebs C., Booker S. J. (2012) RlmN and AtsB as models for the overproduction and characterization of radical SAM proteins. Methods Enzymol. 516, 125–152 [DOI] [PubMed] [Google Scholar]
- 43. Trautwein A. X., Bill E., Bominaar E. L., Winkler H. (1991) Iron-containing proteins and related analogs—complementary Mössbauer, EPR and magnetic-susceptibility studies. Struct. Bond. 78, 1–95 [Google Scholar]
- 44. Beinert H., Holm R. H., Münck E. (1997) Iron-sulfur clusters: nature's modular, multipurpose structures. Science 277, 653–659 [DOI] [PubMed] [Google Scholar]
- 45. Sweeney W. V., Rabinowitz J. C. (1980) Proteins containing 4Fe-4S clusters: an overview. Annu. Rev. Biochem. 49, 139–161 [DOI] [PubMed] [Google Scholar]
- 46. Sakamoto T., Wada H., Nishida I., Ohmori M., Murata N. (1994) Δ9 acyl-lipid desaturases of cyanobacteria. Molecular cloning and substrate specificities in terms of fatty acids, sn-positions, and polar head groups. J. Biol. Chem. 269, 25576–25580 [PubMed] [Google Scholar]
- 47. Perera I. C., Grove A. (2010) Molecular mechanisms of ligand-mediated attenuation of DNA binding by MarR family transcriptional regulators. J. Mol. Cell. Biol. 2, 243–254 [DOI] [PubMed] [Google Scholar]
- 48. Khoroshilova N., Popescu C., Münck E., Beinert H., Kiley P. J. (1997) Iron-sulfur cluster disassembly in the FNR protein of Escherichia coli by O2: [4Fe-4S] to [2Fe-2S] conversion with loss of biological activity. Proc. Natl. Acad. Sci. U.S.A. 94, 6087–6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jervis A. J., Crack J. C., White G., Artymiuk P. J., Cheesman M. R., Thomson A. J., Le Brun N. E., Green J. (2009) The O2 sensitivity of the transcription factor FNR is controlled by Ser24 modulating the kinetics of [4Fe-4S] to [2Fe-2S] conversion. Proc. Natl. Acad. Sci. U.S.A. 106, 4659–4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Crack J. C., Green J., Cheesman M. R., Le Brun N. E., Thomson A. J. (2007) Superoxide-mediated amplification of the oxygen-induced switch from [4Fe-4S] to [2Fe-2S] clusters in the transcriptional regulator FNR. Proc. Natl. Acad. Sci. U.S.A. 104, 2092–2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang B., Crack J. C., Subramanian S., Green J., Thomson A. J., Le Brun N. E., Johnson M. K. (2012) Reversible cycling between cysteine persulfide-ligated [2Fe-2S] and cysteine-ligated [4Fe-4S] clusters in the FNR regulatory protein. Proc. Natl. Acad. Sci. U.S.A. 109, 15734–15739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roche B., Aussel L., Ezraty B., Mandin P., Py B., Barras F. (2013) Reprint of: Iron/sulfur proteins biogenesis in prokaryotes: formation, regulation and diversity. Biochim. Biophys. Acta 1827, 923–937 [DOI] [PubMed] [Google Scholar]
- 53. Wang T., Shen G., Balasubramanian R., McIntosh L., Bryant D. A., Golbeck J. H. (2004) The sufR gene (sll0088 in Synechocystis sp. strain PCC 6803) functions as a repressor of the sufBCDS operon in iron-sulfur cluster biogenesis in cyanobacteria. J. Bacteriol. 186, 956–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shen G., Balasubramanian R., Wang T., Wu Y., Hoffart L. M., Krebs C., Bryant D. A., Golbeck J. H. (2007) SufR coordinates two [4Fe-4S]2+,1+ clusters and functions as a transcriptional repressor of the sufBCDS operon and an autoregulator of sufR in cyanobacteria. J. Biol. Chem. 282, 31909–31919 [DOI] [PubMed] [Google Scholar]
- 55. Yu J., Shen G., Wang T., Bryant D. A., Golbeck J. H., McIntosh L. (2003) Suppressor mutations in the study of photosystem I biogenesis: sll0088 is a previously unidentified gene involved in reaction center accumulation in Synechocystis sp. strain PCC 6803. J. Bacteriol. 185, 3878-3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Frigaard N. U., Li H., Milks K. J., Bryant D. A. (2004) Nine mutants of Chlorobium tepidum each unable to synthesize a different chlorosome protein still assemble functional chlorosomes. J. Bacteriol. 186, 646–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Elhai J., Wolk C. P. (1988) A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene 68, 119–138 [DOI] [PubMed] [Google Scholar]
- 58. Alvey R. M., Biswas A., Schluchter W. M., Bryant D. A. (2011) Effects of modified phycobilin biosynthesis in the cyanobacterium Synechococcus sp. strain PCC 7002. J. Bacteriol. 193, 1663–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.