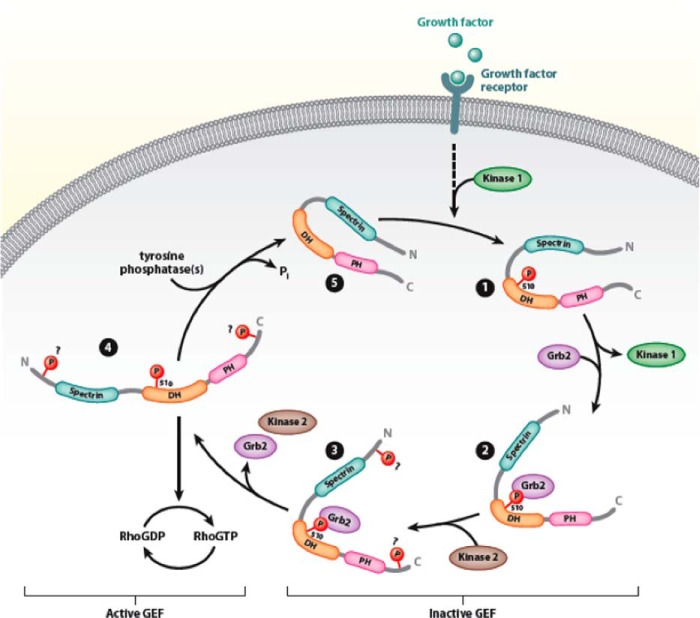
FIGURE 8.
Proposed model of Dbl regulation by tyrosine phosphorylation. In quiescent cells, both intramolecular and intermolecular interactions maintain Dbl in an inactive state (step 5). Growth factor stimulation leads to phosphorylated of Tyr510 (step 1), leading to the recruitment of Grb2 (step 2) and other kinases (step 3) necessary for the phosphorylation of other phosphorylation sites. Full phosphorylation relaxes the autoinhibited structure of Dbl to one that allows high-affinity binding of substrate GTPases and facilitation of nucleotide exchange (step 4). DH, Dbl homology domain; PH, pleckstrin homology domain; N, N terminus; C, C terminus.