Figure 3. ATPase activity of Msh2-Msh3 is modulated by divalent cation and DNA substrate.
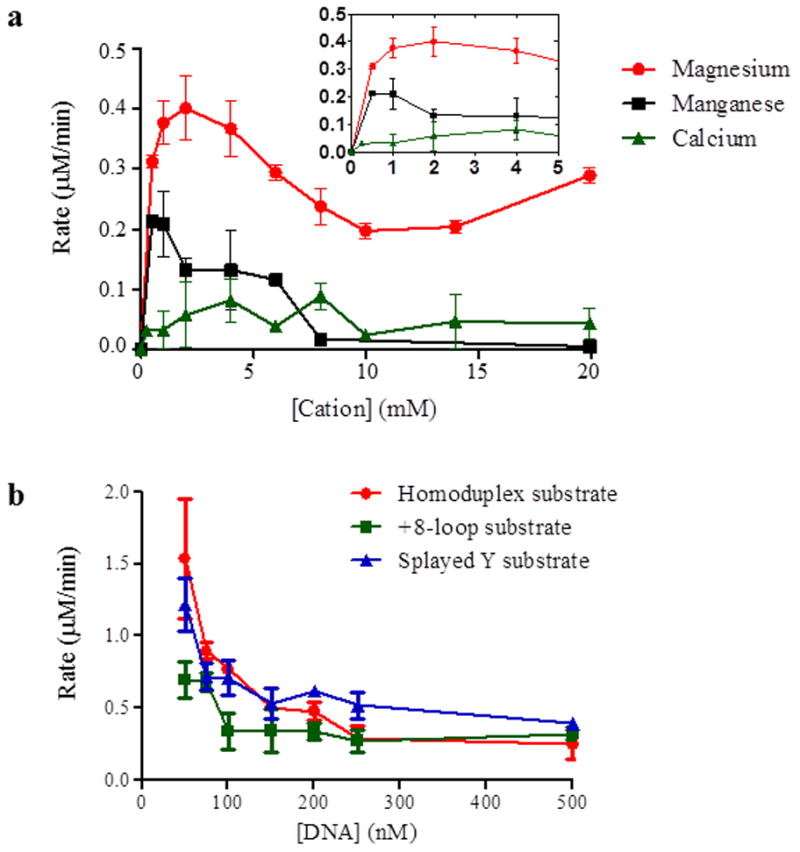
(a) ATPase activity of Msh2-Msh3 is dependent on magnesium. Cation titrations were performed with 100nM Msh2-Msh3 in the presence of 500 nM +8-loop substrate, 1 mM ATP and 100 mM NaCl. Magnesium acetate, manganese chloride and calcium chloride were titrated at the indicated concentrations. The inset shows low cation concentrations at higher resolution. (b) Homoduplex, +8-loop and splayed Y substrates were titrated into an ATPase reaction containing 50 nM Msh2-Msh3, 1 mM ATP and 100 mM NaCl. The DNA titration was started at 50 nM and continued to 500nM DNA substrate. The rate of ATP hydrolysis was plotted against the concentration of DNA. Red circles represent homoduplex DNA, green squares represent +8 loop DNA and blue triangles represent splayed DNA.
