Figure 8. Distinct DNA repair substrates differentially regulate Msh2-Msh3 ATP hydrolytic cycle.
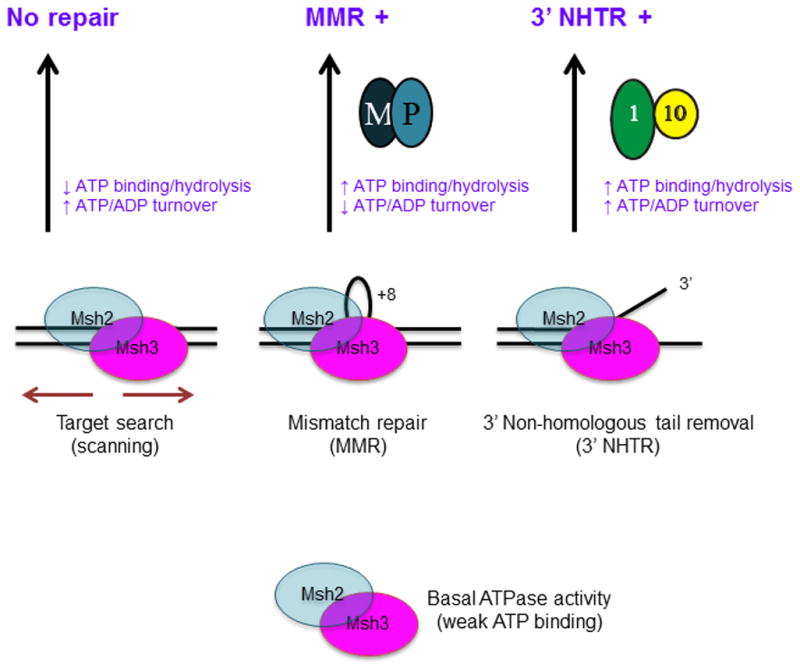
We propose that Msh2-Msh3 shuttles between different free and DNA bound states. The repair mechanism activated depends on the specific conformational changes induced by the different DNA substrates. These conformational changes then induce distinct changes within the ATPase domain, modulating its activities. Increased ATP binding and higher catalytic efficiency by Msh2-Msh3 in the presence of MMR and 3′ NHTR substrates result in repair. Increased ATP hydrolysis (relative to homoduplex) and slowed “nucleotide turnover” (indicated by reduced ATPγS inhibition of ATPase activity relative to homoduplex and 3′ NHTR) in response to the MMR substrate are predicted to be critical for the proper initiation of Msh2-Msh3-mediated MMR, perhaps allowing interactions with Mlh1-Pms1 (M-P). In contrast, 3′ NHTR does not require slowed “nucleotide turnover”, indicating intact interactions between Msh2-Msh3 and Rad1-Rad10 (1–10). Altered ATP binding/hydrolysis by Msh2-msh3Y942A in the presence of homoduplex DNA, indicated by loss of negative cooperativity (Table 3), could promote inappropriate initiation of MMR. Decreased ATP hydrolysis and/or increased nucleotide turnover by Msh2-msh3Y942A in the presence of MMR substrates inactivate MMR, but do not significantly impair 3′ NHTR in vivo [29]. See text for additional details.
