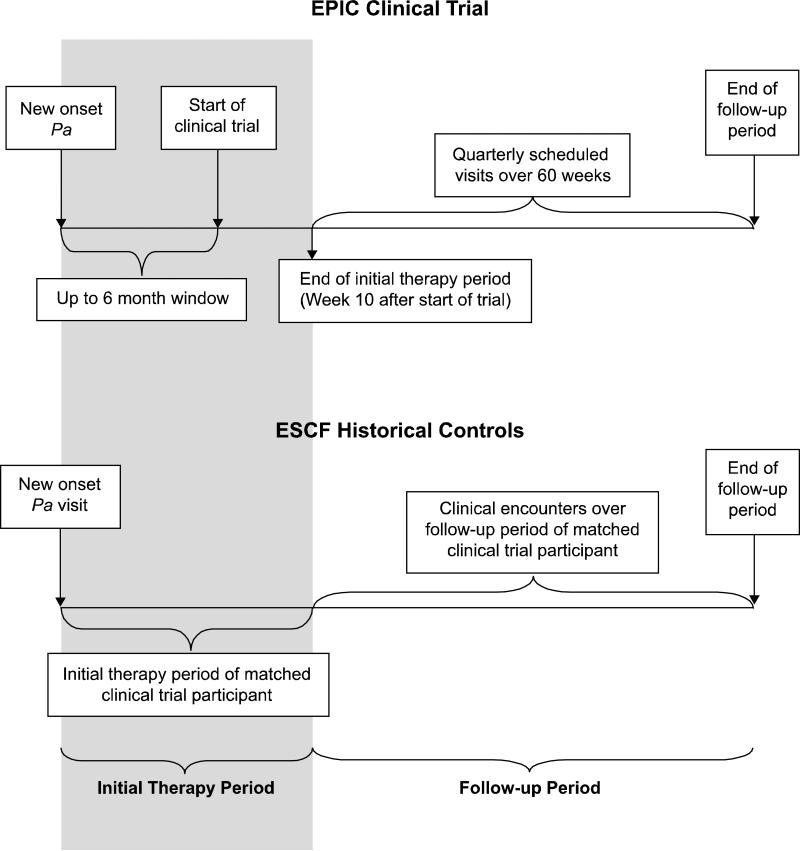
Figure 1. Schematic of Data Collection and Timing.
The initial therapy period for the EPIC clinical trial participants was defined as the time between the Pa qualifying culture and 10 weeks post their baseline visit in the clinical trial. The clinical trial allowed up to a 6-month window between the new onset Pa that defined eligibility and the baseline randomization visit and during this time, participants were allowed limited anti-pseudomonal antibiotics.(20) For the historical controls, the length of the initial therapy period for each control was determined based on that of their matched clinical trial participant. The follow up period for each clinical trial participant was defined as the time between the end of the initial therapy period (approximately 10 weeks into the clinical trial) and the day of their final study visit at approximately 70 weeks post-randomization. The follow up times were derived for each clinical trial participant and similarly used to derive matched follow up periods for the controls.