Figure 2. Redox cycle of Hsp33.
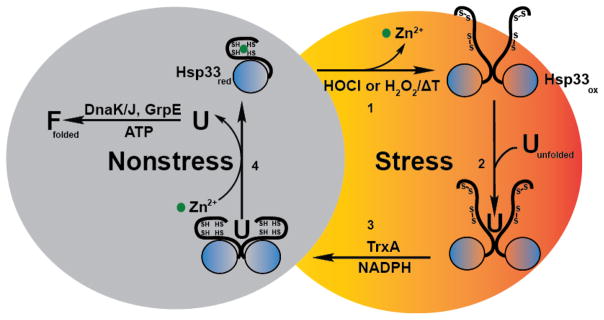
Under non-stress conditions, the heat shock protein Hsp33 is reduced and chaperone-inactive. All four highly conserved cysteines coordinate one zinc ion with high affinity. (1) Upon exposure to bleach (HOCl) or hydrogen peroxide (H2O2) in combination with elevated temperatures, Hsp33’s cysteines form two disulfide bonds, causing the zinc to be released. Loss of zinc binding in combination with disulfide bond formation leads to the partial unfolding of Hsp33, the required step for Hsp33’s activation as a chaperone. Two partially unfolded monomers then associate to form a chaperone-active Hsp33 dimer. (2) Once activated, Hsp33 stabilizes oxidatively unfolding proteins and forms stable client protein – chaperone complexes. (3) Upon return to non-stress conditions, the thioredoxin system reduces Hsp33’s disulfide bonds, generating a chaperone-active reduced dimer – client protein complex. (4) In the last step, the client protein is transferred to the DnaK/DnaJ/GrpE system for productive refolding, and Hsp33 dissociates into its monomeric, inactive state.
