Figure 5. Peroxiredoxin – An Enzyme with Multiple Personalities.
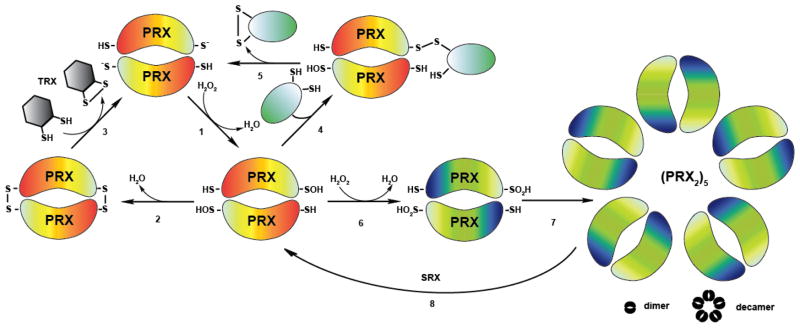
(1) Peroxiredoxins (orange) are responsible for the breakdown of hydrogen peroxide. In this process, their peroxidatic cysteines become oxidized to sulfenic acids. (2) Intermolecular disulfide bond formation with the resolving cysteines in Prx follows. (3) The disulfide bond is subsequently reduced by the Trx system. (4) In an alternative pathway, oxidized peroxiredoxin interacts with the thiol group of a reduced client protein (e.g. Yap1p), forming an intermolecular disulfide bond. (5) This disulfide bond is then resolved by a thiol-disulfide exchange reaction, yielding in an intramolecular disulfide bond within the client protein and reduced Prx. (6) High levels of peroxide lead to the formation of sulfinic acid at the active site cysteine and the inactivation of the peroxidase function. (7) Overoxidation triggers the assembly of peroxiredoxin into higher molecular weight structures, which exert chaperone function in vitro. (8) ATP-dependent sulfiredoxin (SRX) reduces the sulfinic acid in peroxiredoxin.
