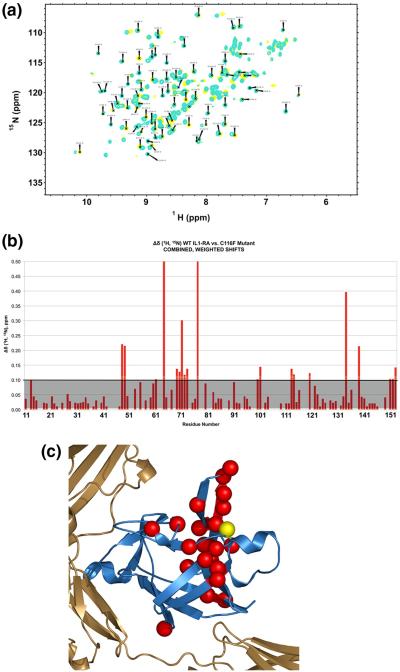
Fig. 3.
Characterization of the effects of C116F mutation on the protein structure with NMR. (a) An overlay of the HSQC spectra for WT (cyan) and mutant (yellow) IL-1Ra. The global pattern of chemical shifts and dispersion of the backbone resonances in the 1H–15N HSQC spectra of WT IL-1Ra and C116F indicate a similar overall global fold. (b) Graphic of the combined weighted proton/nitrogen chemical shift changes in IL-1Ra as a result of the C116F mutation. The changes greater than 0.1 are as indicated above the grey shaded area. (c) Significant chemical shift differences observed in the free protein upon mutation are indicated by red spheres and mapped back onto the molecule. The yellow sphere indicates the site of mutation.