Fig. 7.
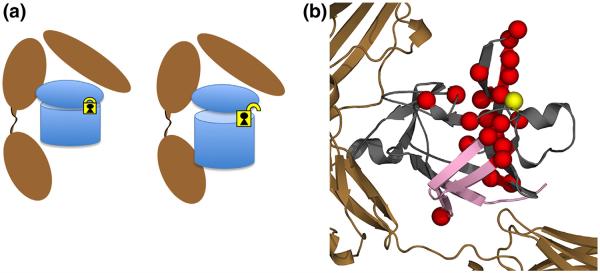
Schematic of proposed interaction of the C116F mutant with respect to receptor binding and subsequent signaling as a result of mutation. (a) The agonist-like behavior from the introduction of a phenylalanine at position C116 creates a subtle rearrangement of the residues around area of mutation (yellow lock), disrupting interactions related to a potential intramolecular disulfide. This unlocks the pinned hairpin cap from the β-barrel, allowing for engagement of the B-site at the opposite end of the protein, eliciting a signal response. (b) Molecular representation of both the chemical shift NMR results on the free ligand (red spheres) and DXMS (pink strands) of the receptor complex. When bound to the receptor, significant destabilization in HDX is observed in β-barrel strands 1, 8, 9, and 12 in the C116F mutant protein (pink strands). These results indicate that the mutant protein adjusts upon binding such that it can now engage both sites A and B (Fig. 1) as it becomes signaling competent. This long-range control of a binding interface is distinct from the classic allosteric mechanism.
