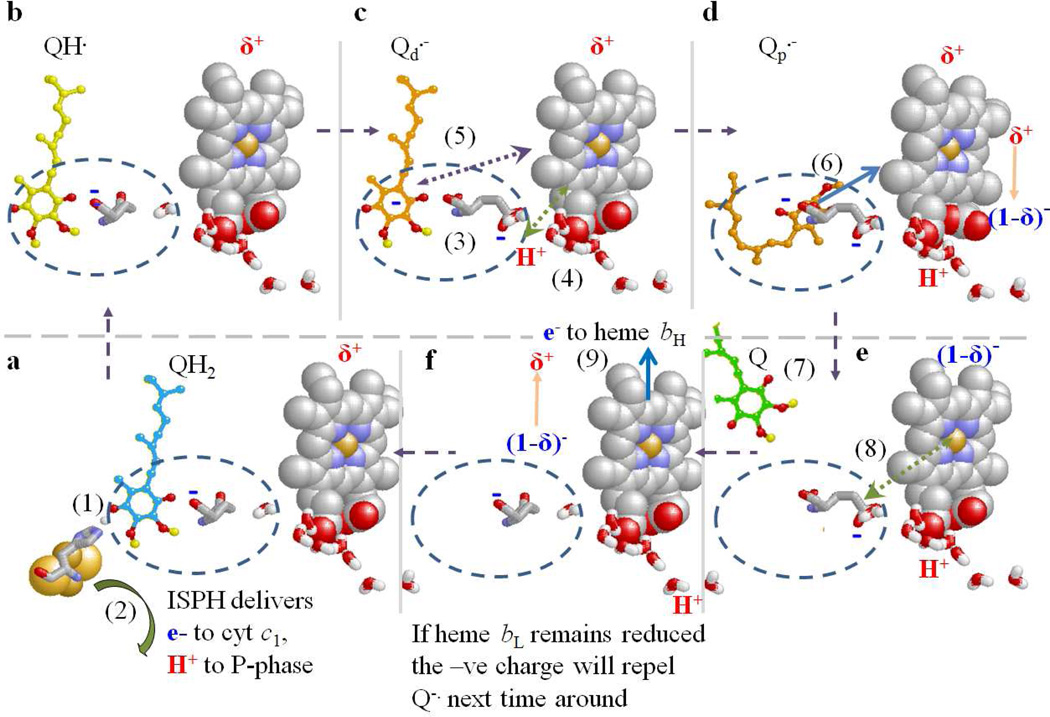
Figure 8. Scheme to show possible mechanistic features of the Qo-site ballet.
a. Before the 1st electron transfer, the ES1-complex forms in the distal volume of the Qo-site, stabilized by a strong H-bond to His-161 of ISPox. E295 may H-bond weakly to QH2. Heme bL is in the oxidized form (with local field of δ+ charge). An electron and H+ are delivered to ISPox to form ISPH (1). Immediately after the 1st electron transfer, the ISPH rotates away (2) to deliver its electron to cyt c1 and release the H+ to the P-phase.
b. This leaves the neutral SQ (QH.), H-bonded to E295 carboxylate in the ES2-complex. Heme bL is still oxidized, and available as acceptor for the electron from QH. (or Q.-), but the low occupancy, and low value for k2d (~103 s−1) means the rate is slow.
c. The H+ is transferred to the E295 carboxylate, to form the SQ anion (Q.-), and E295 (3), now in carboxylic form, rotates to open up the proximal volume. The sidechain makes a H-bond to the water chain leading to the P-side aqueous phase. The δ+ field around heme bL lowers the pK, allowing release of the H+ (4), which transfers down the water chain, leaving E295 in the carboxylate form. The δ+ field also attracts Q.- (5), which migrates closer to heme bL, increasing the rate constant (k2p ~109 s−1).
d. The electron from Q.- transfers rapidly to heme bL (6), and the quinone is free to migrate back to the distal volume, and exit the site (7). Reduction of heme bL changes the charge by 1, and the field changes transiently to (1-δ)−.
e. The field is felt by the carboxylate of E295 (8), and the coulombic force flips it back to its initial position.
f. If heme bH is oxidized, the electron is transferred (9), and the field associated with heme bL returns to its initial δ+ charge. The site is now vacant, and available to accept a QH2 for the next turnover. If heme bL remains reduced (after the second turnover), the (1-δ)− field serves to repel the Q.- formed after the next cycle (reactions (1) – (3) representing a partial third turnover).