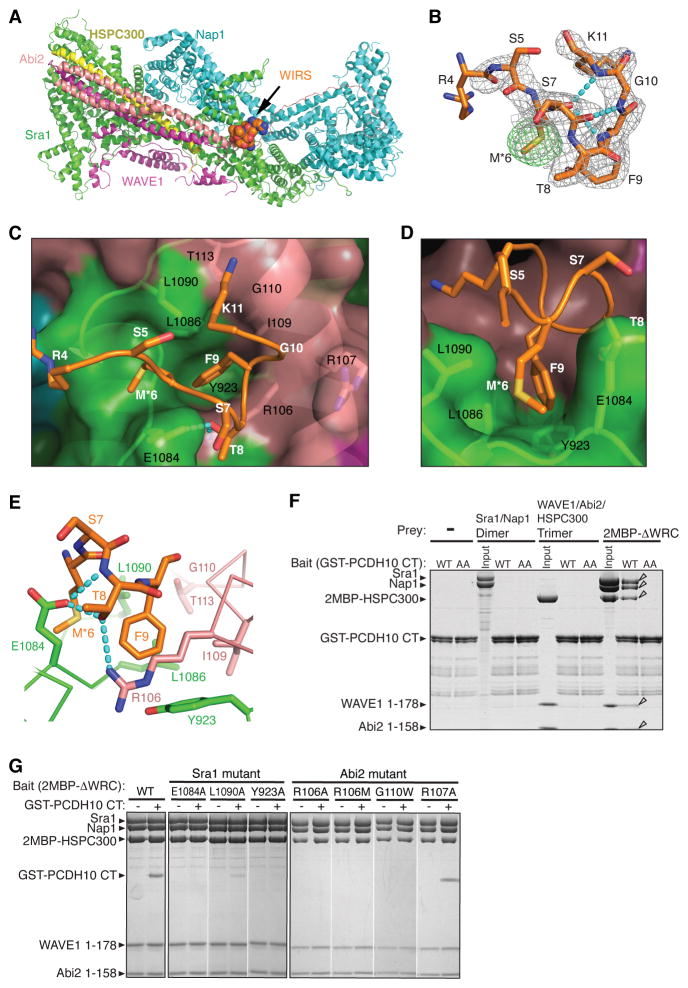
Figure 2. WIRS Binds to a Composite Surface Formed by Sra1 and Abi2.
(A) Structure of the WIRS/WRC complex (Sra1: green; Nap1: cyan; HSPC300: yellow; Abi2: pink; WAVE1: magenta; WIRS peptide: spheres).
(B) 2Fo-Fc electron density map (grey mesh, 1.2 σ) and anomalous scattering map (green mesh, 4 σ) around the WIRS peptide. Cyan dotted lines show intra-peptide hydrogen bonds.
(C and D) Top and side views of a semi-transparent WRC surface (key side chains shown under the surface, black labels) with WIRS peptide (white labels).
(E) WRC-WIRS interactions; dotted lines show intermolecular hydrogen bonds.
(F and G) Commassie blue stained SDS-PAGE gels of eluted proteins are shown. In (F), immobilized GST-PCDH10 CT wild type (WT) or mutant (AA for T1002A/F1003A) selectively retained WRC but not indicated sub-complexes. Open triangles indicate bound proteins. In (G), immobilized 2MBP-ΔWRC mutants selectively retained GST-PCDH10 CT.