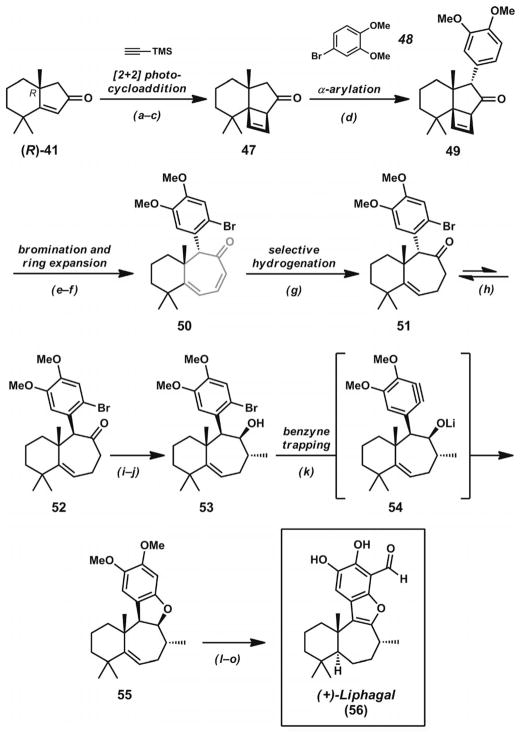
Scheme 8.
Total synthesis of liphagal. Reaction conditions: a) TMS-acetylene, UV-B lamps, acetone. b) BF3·OEt2, CH2Cl2. c) TBAF, THF (68% yield, three steps). d) [Pd(P(tBu)3)2] (5 mol-%), 4-bro-moveratrole, NaOtBu, THF, microwave irradiation, 120 °C (67% yield). e) Br2 (1.8 equiv.), CHCl3 (65% yield). f) Microwave irradiation, o-dichlorobenzene, 250 °C (68% yield). g) PtO2 (20 mol-%), H2 (1 atm), EtOAc (69% yield). h) NaOMe, MeOH, 65 °C (78% yield, three cycles). i) LDA, THF, −78→0 °C; then CH3I, −78→0 °C (68% yield). j) DIBAL, PhCH3 (91% yield). k) LDA (3 equiv.), THF, −20 °C (83% yield). l) Pd/C (19 mol-%), H2 (1 atm), EtOH, 21 °C (97% yield). m) NO+BF4−, CH3CN, 0 °C (70% yield). n) nBuLi, TMEDA, THF, 0 °C; then DMF, 0→21 °C (70% yield). o) BI3, CH2Cl2 (45 % yield).