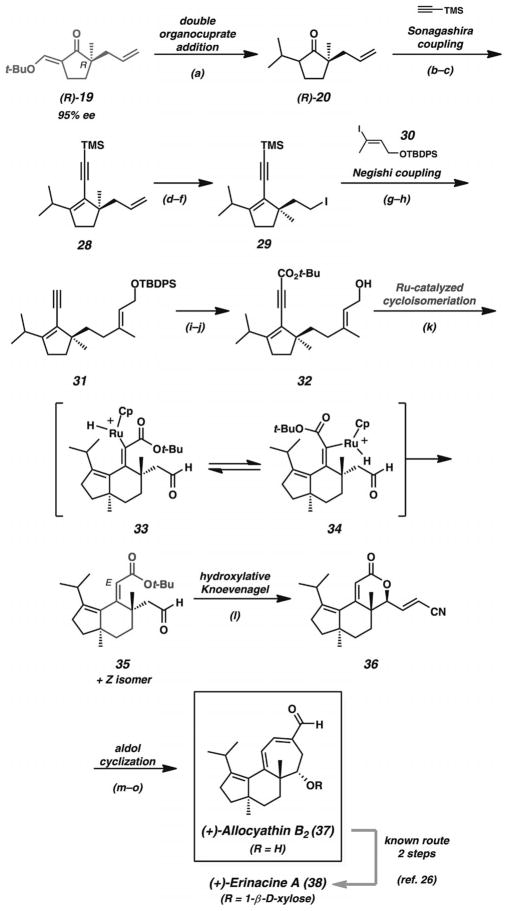
Scheme 6.
Total synthesis of allocyathin B2. Reaction conditions: a) (CH3)2CuLi, Et2O, −20 °C (89% yield). b) LDA, THF, −78→0 °C; then PhN(Tf)2 (96% yield). c) [Pd2(dba)3]·CHCl3 (2.5 mol-%), PPh3 (20 mol-%), CuI (5 mol-%), TMS-acetylene, nBuNH2, 50 °C (85% yield). d) OsO4 (1 mol-%), NMO; then NaIO4 (87% yield). e) NaBH4, MeOH (94% yield). f) PPh3, I2, imidazole (97% yield). g) tBuLi, ZnCl2, THF, −78 °C →r.t.; then [Pd(PPh3)4] (5 mol-%), vinyl iodide 30. h) K2CO3, MeOH (74% yield, two steps). i) nBuLi, THF, −78 °C; then Boc2O, −78 →r.t. (99% yield). j) TBAF, THF (52–55% yield). k) CpRu(CH3CN)3PF6 (20 mol-%), DMF (1 equiv.), butan-2-one, room temp. (48% yield of 35, or 55% combined yield, 6.7:1 E/Z ratio). l) PhS(O)CH2CN, piperidine, PhH (75% yield). m) Pd/C (10%), EtOAc, H2 (1 atm) (83% yield). n) DIBAL, CH2Cl2, −78 °C. o) KOH, MeOH, 60 °C (51% yield, two steps).