FIGURE 1.
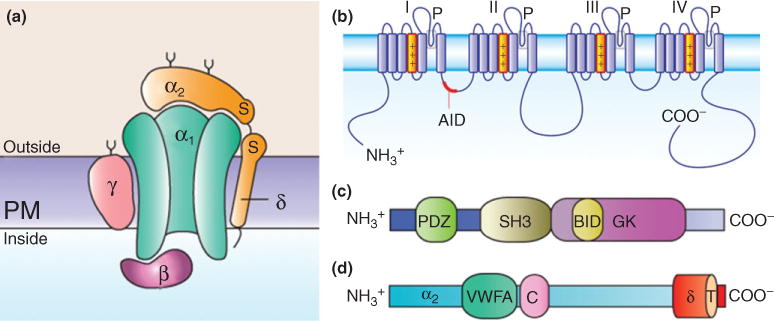
Subunit composition of high-voltage-activated Ca2+ channels and structural domains of the auxiliary subunits. (a) Channel complex is composed of the pore-forming CaVα1 and the auxiliary CaVα2δ, CaVβ, and CaVγ subunits. The CaVα2δ and CaVγ subunits contain transmembrane domains, whereas CaVβs are intracellular. (b) The CaVα1 subunits consist of four transmembrane domains (I–IV) and the linker joining I and II encompasses the α-interaction domain (AID). (c)The CaVβ subunits are formed by three conserved domains: PSD95/Dlg1/ZO-1 (PDZ), Scr homology 3 (SH3), and guanylate kinase (GK). The CaVβ subunit interaction domain (BID) is one of the regions involved in the interaction of the protein with the CaVα1 subunit. (d) The CaVα2δ subunits consist of α2 (blue), which is an extracellular subunit, disulphide-bonded to the δ subunit (orange), which is membrane-associated. The approximate positions of the vWA domain and the two bacterial chemosensory domains (Cache; C) are also indicated. The sites of interaction between the CaVα1 subunit and the CaVα2δ subunit are unknown.
