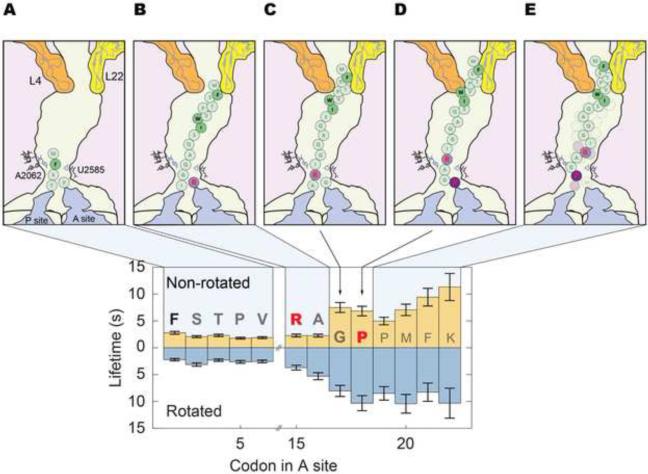
Figure 7. SecM induced stalling is a dynamic phenomenon that leads to a region of significantly reduced elongation rates.
(A) The ribosome translates the SecM sequence normally over the first 13 codons. (B) The peptide then interacts with the constriction point in the exit tunnel, formed by the large subunit proteins L4 and L22. This compacts and increases the mechanical stress on the peptide. The effect propagates downstream via the peptide or the tunnel, leading to reduced translocation rates. (C) Compaction of the peptide positions Arg15 to interact with the exit tunnel entrance to remodel tRNA geometry in the peptidyl transferase center (PTC), slowing down peptide bond formation. (D) This opens a window for Pro18 to lock the SecM peptide in conformations that induces a high level of mechanical stress leading to a heavily remodeled PTC geometry. (E) Elongation rates are greatly slowed over the next 4~5 codons past the terminal proline, leading to stalling that is stable up to an hour.