Abstract
Ondansetron is a 5-HT3 receptor antagonist that is an effective anti-emetic in cats. The purpose of this study was to evaluate the pharmacokinetics of ondansetron in healthy cats. Six cats with normal complete blood count, serum biochemistry, and urinalysis received 2 mg oral (mean 0.43 mg/kg), subcutaneous (mean 0.4 mg/kg), and intravenous (mean 0.4 mg/kg) ondansetron in a cross-over manner with a 5-day wash out. Serum was collected prior to, and at 0.25, 0.5, 1, 2, 4, 8, 12, 18, and 24 h after administration of ondansetron. Ondansetron concentrations were measured using liquid chromatography coupled to tandem mass spectrometry. Noncompartmental pharmacokinetic modeling and dose interval modeling were performed. Repeated measures anova was used to compare parameters between administration routes. Bioavailability of ondansetron was 32% (oral) and 75% (subcutaneous). Calculated elimination half-life of ondansetron was 1.84 ± 0.58 h (intravenous), 1.18 ± 0.27 h (oral) and 3.17 ± 0.53 h (subcutaneous). The calculated elimination half-life of subcutaneous ondansetron was significantly longer (P < 0.05) than oral or intravenous administration. Subcutaneous administration of ondansetron to healthy cats is more bioavailable and results in a more prolonged exposure than oral administration. This information will aid management of emesis in feline patients.
INTRODUCTION
Ondansetron is a 5HT3 receptor antagonist anti-emetic drug that was developed to treat chemotherapy-induced emesis in humans (Roila & Del Favero, 1995). It has been demonstrated to be an effective anti-emetic in cats, blocking dexmedetomadine-induced emesis, and has been shown to decrease feline intestinal motility (Santos et al., 2011; Wang et al., 2011). Clinically it is used in the treatment for nausea and vomiting associated with chemotherapy, acute vomiting such as pancreatitis, as well as chronic illness such as chronic kidney disease (CKD) (Ljutic et al., 2002; Trepanier, 2010; O’Connor & Corcoran, 2012). Empirical dosing in cats has previously been described as 0.5 mg/kg twice daily, oral or injectable (Trepanier, 2010); however, pharmacokinetic studies in cats have not been performed to confirm these recommendations. Bioavailability varies widely between species, varying from 4 to 62% orally in rats and humans, respectively; thus, additional information about the effects of route of administration on pharmacokinetics in cats would be beneficial (Yang & Lee, 2008). The purpose of this study was to perform pharmacokinetic analysis of ondansetron in cats to allow more effective therapeutic administration for this species and to explore which dose route would be most amenable to repeated administration in chronically ill animals.
MATERIALS AND METHODS
Animals
Six healthy purpose-bred cats were utilized for the pharmacokinetic study after undergoing a physical exam, complete blood count, chemistry and urinalysis to rule out systemic disease. The cats were all 1.5 years of age and included three spayed females and three neutered males. All portions of the project were approved by the Institutional Care and Use Committee at Colorado State University.
Dosing and sampling
A jugular catheter was placed under ketamine/butorphanol sedation [20 mg ketamine/cat intravenous (i.v.), 0.1 mg/kg butorphanol i.v.] 16 h prior to study initiation to allow for ease of sample collection. Each cat received 2 mg oral (mean 0.43 mg/kg), subcutaneous (SQ) (mean 0.4 mg/kg), and i.v. (mean 0.4 mg/kg) ondansetron (injectable: West/Ward Pharmaceuticals, Eatontown, NJ, USA; oral: Actavis Inc., Morristown, NJ, USA) with at least a 5-day wash out period between cross-over to the other drug formulation. The 2-mg dose was selected for analysis because it is commonly prescribed due to the availability of a 4-mg oral tablet, and because it fell between the previously reported effective dose of 0.22 mg/kg and the previous recommended dose of 0.5 mg/kg (Sedlacek et al., 2008; Trepanier, 2010; Santos et al., 2011). The administered oral dose was calculated by weighing each 4-mg tablet, halving the tablet, and weighing the administered segment. Blood samples were collected prior to, and at 0.25, 0.5, 1, 2, 4, 8, 18, and 24 h after administration of 2 mg of oral, SQ or i.v. ondansetron. Serum was collected by centrifugation immediately after clot formation and frozen in aliquots at −80 °C until analysis.
Materials
Ondansetron and the internal standard, zolpidem, were obtained from Sigma (St. Louis, MO, USA). All other chemicals and solvents used in the analysis were of reagent or higher quality and collected from Fisher’s Scientific (Pittsburgh, PA, USA).
Standard curve and sample extraction
A standard curve and QCs were prepared in the following manner: Initial stocks of ondansetron and zolpidem were prepared in 1:1 methanol/Milli-Q water (1 mg/mL). A standard curve of ondansetron was then prepared ranging from 2.5 to 2000 ng/mL in 1:1 methanol/Milli-Q water. Ten micro litre of each appropriate standard was then added to 100 µL of blank serum collected from cats of similar age and fortified with 10 µL internal standard (zolpidem at 1000 ng/mL). QCs of low, medium and high concentrations (5, 50, and 500 ng/mL) were prepared in a similar manner.
Ten-micro litre internal standard and 10-µL 1:1 methanol/Milli-Q water were added to 100 µL of each unknown sample. All samples were then extracted with 500 µL ethyl acetate, vortex mixed for 10 min, centrifuged at 14 000 g for 10 min, and 450 µL of the organic phase was transferred to fresh 2-mL micro-centrifuge tubes. Samples were concentrated to dryness on a speed vacuum then reconstituted in 200 µL of 80:20 [v: v], 10 mm ammonium acetate with 0.1% acetic acid: acetonitrile (ACN). Finally, samples were transferred to glass autosampler vials for injection onto the HPLC system.
Mass spectrometry
Mass spectra were obtained with a MDS Sciex 3200 Q-TRAP triple quadrupole mass spectrometer (Applied Biosystems, Inc., Foster City, CA, USA) with a turbo ionspray source interfaced to an Agilent 1200 Series Binary Pump SL HPLC system (Santa Clara, CA, USA). Samples were chromatographed with an XBridge Phenyl, 2.5 µm, 4.6 × 50 mm column (Waters Corporation, Milford, MA, USA) protected by a C18 guard cartridge, 4.0 × 2.0 mm (Phenomenex, Torrance, CA, USA). An LC gradient was employed with mobile phase A consisting of 10 mm ammonium acetate with 0.1% acetic acid and mobile phase B consisting of acetonitrile. Chromatographic resolution was achieved by increasing mobile phase B linearly from 25 to 98% from 0.5 to 3 min, maintaining at 98% from 3 to 4 min, decreasing linearly from 95 to 25% from 4 to 4.5 min, followed by re-equilibration of the column at 25% B from 4.5 to 6 min. The LC flow rate was 1.1 mL/min, the sample injection volume was 10 µL, and the analysis run time was 6 min.
Operating in positive ion electrospray mode, source-dependent parameters were optimized as follows: turbo ionspray temperature, 550 °C; ion spray voltage, 5500 V; curtain gas, N2, (CUR), 20 units; collision gas, N2, (CAD), low; nebulizer gas, N2, 60 units; and auxiliary gas, N2, 60 units. The compound-dependent parameters for ondansetron and zolpidem, respectively, were optimized as follows: declustering potential (DP), 40 and 64 V; entrance potential (EP), 10.6 and 9.7 V; collision energy (CE), 37 and 47 V; collision cell entrance potential (CEP), 20 and 32 V; collision cell exit potential (CXP), 5.1 and 4.0 V. The predominant product ion for ondansetron was m/z 170.1. Samples were quantified by internal standard reference method in the MRM mode monitoring ion transitions m/z 294.2 → 170.1 for ondansetron and m/z 308.2 → 235.1 for the internal standard, zolpidem. The dwell times for each ion transition were 250 ms. Q1 and Q3 were both operated in unit resolution mode.
Data analysis
Quantitation of ondansetron was based on linear standard curves in spiked blank serum using the ratio of ondansetron peak area to zolpidem peak area and 1/x2 weighting of linear regression. Parameters for the assessment of assay performance were calculated as follows:
Noncompartmental and compartmental modeling was used for the calculation of pharmacokinetic (PK) parameters using Phoenix® WinNonlin® software, version 6.3 (Pharsight Corp., Cary, NC, USA).
Dosing interval modeling
Prediction of ondansetron in cat serum following dosing at 0.5 and 1 mg/kg via i.v., oral and SQ dosing utilizing varied dosing intervals was carried out using nonparametric superposition based on concentration data from single dose studies for each individual cat. The extrapolation of the single dose data was carried out in a model-independent manner with the following assumptions: (i) each dosing interval is independent of the others; (ii) drug absorption is constant across doses; (iii) drug elimination (CL) is constant across doses; and (iv) serum concentration is linear with dose across the dose utilized in the single dose study and those extrapolated. Values represent the mean ± standard deviation of simulations for data from each individual cat used in the single dose studies. All modeling was carried out using Phoenix™ WinNonlin® 6.3 on a PC-based computer system.
The pharmacologically active serum concentration for ondansetron preventing emesis was estimated from data presented in Santos et al. where a dose of 0.22 mg/kg i.m. ondansetron given concurrently with dexmedetomidine blocked vomiting but a dose given 30 min prior did not (Santos et al., 2011). Thus, it was estimated that levels of ondansetron at approximately 15 min postdosing were effective whereby levels at 45 min postdosing would not be effective at this dose. Serum levels for ondansetron were then estimated at the 0.22 mg/kg dose based on the current PK data assuming that SQ and i.m. dosing routes would show similar serum level vs. time profiles.
Statistical analysis
Pharmacokinetic parameters were compared between administration routes using repeated measures anova in Prism software (Prism 5; GraphPad, La Jolla, CA, USA). Parameters assessed included time to maximum serum concentration (tmax), maximum serum concentration (Cmax), elimination half-life (t1/2 λ), area under the curve (AUC), volume of distribution (Vz), and clearance (CL). Pharmacokinetic parameters for SQ and oral dosing were adjusted for bioavailability prior to being compared with i.v. dosing parameters (CL and Vz). Bioavailability was compared between oral and SQ dosing using a Wilcoxon sign rank test in Prism software. For all analyses, a P-value < 0.05 was considered statistically significant.
RESULTS
Chromatography
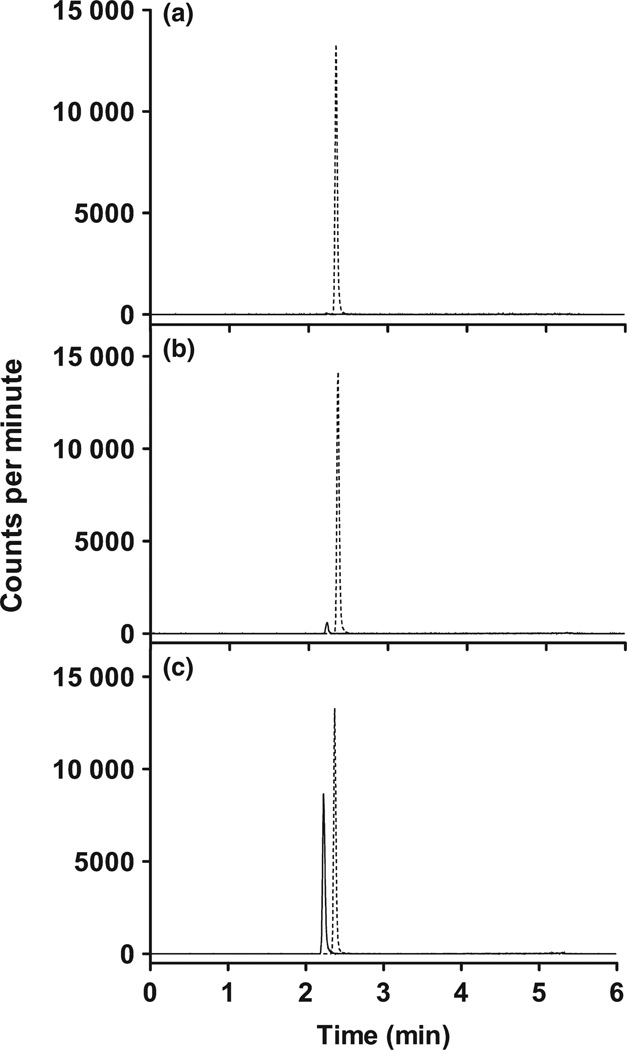
Representative chromatographs of ondansetron measured in cat serum are shown in Fig. 1. Ondansetron and zolpidem eluted 2.2 and 2.4 min, respectively. Peaks were monitoring ion transitions m/z 294.2 → 170.1 for ondansetron and m/z 308.2 → 235.1 for zolpidem. No interfering peaks were detected at the monitored ion transitions in extracted serum. Representative chromatographs, optimized for peak shape, observed for standards (Fig. 1a,b), and from a sample collected 2-h post-SQ dosing (Fig. 1c) in cat serum are shown.
Fig. 1.
Representative chromatographs of ondansetron measure in cat serum. Representative chromatographs, optimized for peak shape, observed for standards (a and b), and from a sample collected 2-h post-SQ dosing (c) in cat serum are shown.
Linearity and LLOQ
Standard curves in spiked cat serum were linear over the range of 2.5–2000 ng/mL. The linearity of the curves was greater than r2 = 0.99 using 1/x2 weighting. As measured in cat serum, the lower limit of quantitation (LLOQ), based on analytical method validation guidelines (Bansal & DeStefano, 2007), was determined to be 2.5 ng/mL in serum. An injection volume of 10 µL allows for quantitation of 11.25 pg per sample injection using the liquid–liquid extraction and analysis described.
Precision and accuracy
The precision and accuracy of ondansetron measurement were assessed in cat serum (Table 1). The accuracy of within-run ondansetron measurements at 5, 50 and 500 ng/mL ranged from 96.4 to 97.1% with the precision (as measured by RSD) ranging from 2.6 to 6.2%. The between-run accuracy at concentrations ranging from 2.5 to 2000 ng/mL in cat serum was from 86.5 to 99.7% and the precision from 2.4 to 9.8%. Accuracy and precision measurements were within the minimum accuracy (85%) and precision (±20%) ranges for acceptable analytical method validation (Bansal & DeStefano, 2007).
Table 1.
Precision and accuracy for ondansetron assay in cat serum within and between runs
| Within representative run (n = 4) |
Between Runs (n = 4) |
|||||
|---|---|---|---|---|---|---|
| Concentration* (ng/mL) | Observed | Accuracy | Precision (RSD %) | Observed | Accuracy | Precision (RSD %) |
| 2.5 | 2.44 ± 0.24 | 97.5 | 9.8 | |||
| 5 | 5.15 ± 0.32 | 97.1 | 6.2 | 5.02 ± 0.16 | 99.7 | 3.1 |
| 10 | 9.88 (9.56, 10.2)† | 98.8 | 4.6 | |||
| 50 | 50.5 ± 1.3 | 99.1 | 2.6 | 52.3 ± 3.3 | 95.5 | 6.3 |
| 100 | 104 ± 3 | 96.4 | 2.8 | |||
| 250 | 255 ± 19 | 98.0 | 7.5 | |||
| 500 | 482 ± 20 | 96.4 | 4.2 | 486 ± 22 | 97.2 | 4.5 |
| 1000 | 952 ± 23 | 95.2 | 2.4 | |||
| 2000 | 1730 ± 61 | 86.5 | 3.5 | |||
Values are concentration in serum (100 µL) that was extracted for analysis.
Two 10 ng/mL standards failed their respective batches. The two that passed are presented.
Drug administration
All cats tolerated i.v. administration of ondansetron, one cat objected to oral administration and one cat reacted to SQ administration by jumping and vocalizing. It was subjectively noted that all cats were unusually quiet (in contrast to their normal boisterous behavior) for approximately 30 min after both i.v. and subcutaneous administration.
Pharmacokinetics
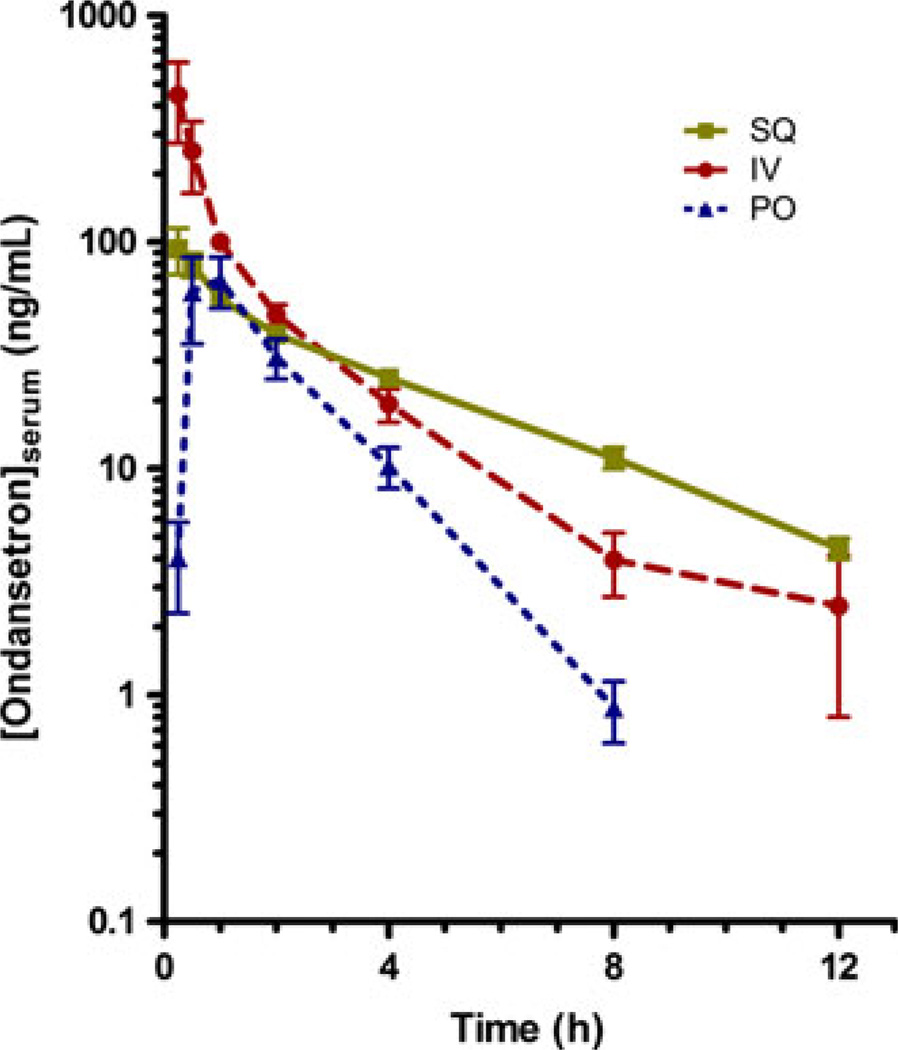
Pharmacokinetic parameters for i.v., oral and SQ ondansetron are summarized in Table 2. Drug concentration curves are illustrated in Fig. 2. The t1/2 λ of SQ ondansetron was significantly longer when compared to i.v. or oral administration (P < 0.05). The tmax of i.v. and SQ ondansetron were similar but the tmax of oral ondansetron was significantly longer than either injectable formulation (P < 0.05). There was a statistically significant difference in the AUC of i.v. ondansetron in comparison with oral (P < 0.05). The Vz of i.v. and oral ondansetron were similar but the Vz of SQ ondansetron was significantly higher than other formulations (P < 0.05). No significant difference in Cmax or CL was found between routes of administration. Oral bioavailability of ondansetron was significantly less than SQ bioavailability when compared using a Wilcoxon sign rank test. (P = 0.03).
Table 2.
Pharmacokinetic parameters for intravenous, oral, and subcutaneous ondansetron administered to healthy cats
| i.v. |
Oral |
SQ |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pharmacokinetic parameter | Median | Range | Mean ± SD | Median | Range | Mean ± SD | Median | Range | Mean ± SD |
| Cmax (ng/mL) | 274 | 228–1310 | 447 ± 426 | 92.7 | 12–143 | 87 ± 54 | 87.7 | 50.9–172 | 98 ± 48 |
| Tmax *,† (h) | – | – | – | 1.0 | 0.5–1 | 0.8 ± 0.3 | 0.4 | 0.25–0.5 | 0.4 ± 0.1 |
| Half-life‡,† (h) | 1.6 | 1.4–3.0 | 1.8 ± 0.6 | 1.1 | 0.9–1.6 | 1.2 ± 0.3 | 3.1 | 2.6–3.8 | 3.2 ± 0.5 |
| AUC∞* (ng/mL·h) | 415 | 433–740 | 454 ± 154 | 148 | 38–252 | 156 ± 79 | 312 | 237–369 | 311 ± 53 |
| Vz (L) | 28.a5 | 23–70 | 29.0 ± 15.3 | – | – | – | – | – | – |
| Vz/F (L)*,† | – | – | – | 24.7 | 17.3–84.3 | 35.7 ± 25.4 | 102.4 | 45.2–160.5 | 99.3 ± 44.9 |
| CL (L/h/kg) | 0.9 | 0.6–1.4 | 0.9 ± 0.3 | – | – | – | – | – | – |
| CL/F (L/h/kg) | – | – | – | 0.9 | 0.64–2.5 | 1.2 ± 0.7 | 0.9 | 0.5–1.6 | 1.0 ± 0.4 |
| Bioavailability† | – | – | – | 0.36 | 0.11–0.5 | 0.32 ± 0.15 | 0.70 | 0.41–1.0 | 0.75 ± 0.28 |
Parameters that are significantly different (P < 0.05) are denoted with (*i.v. vs. p.o.; †p.o. vs. SQ; ‡i.v. vs. SQ).
Fig. 2.
Drug concentration curves for ondansetron administered to healthy cats in a cross-over manner via three routes; i.v., oral, and subcutaneous. Subcutaneous administration sustains statistically significantly higher concentration over time with longer plasma elimination half-life (P < 0.05).
Dose interval modeling
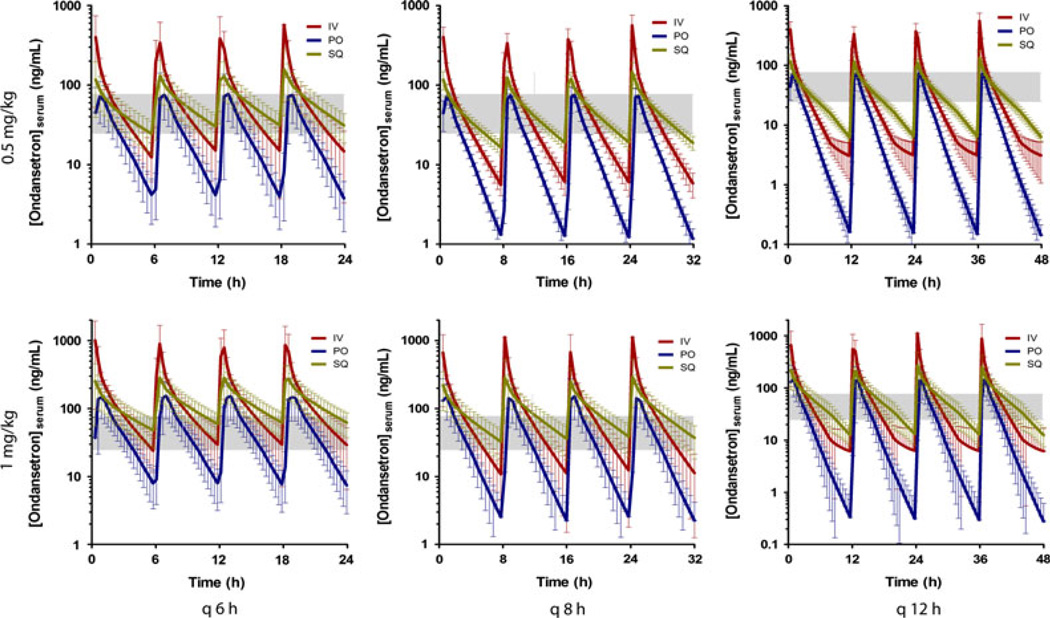
Dose interval modeling is illustrated in Fig. 3. Based on this model, twice daily administration of ondansetron at 0.5 mg/kg by any route resulted in minimal time within the proposed therapeutic range. SQ administration maintains steadier serum concentrations for longer than other routes. Increased dose or frequency resulted in increased time within the therapeutic range.
Fig. 3.
Dose interval modeling of 0.5 and 1 mg/kg ondansetron administered q 6, 8, and 12 h based on data from all six cats. The shaded area represents the estimated effective serum concentration of 25–75 ng/mL.
DISCUSSION
This study examined the pharmacokinetics of i.v., SQ and oral ondansetron in healthy cats, which has not previously been assessed. Bioavailability of ondansetron in cats was 32% (oral) and 75% (subcutaneous). The t1/2 λ of SQ ondansetron was effectively significantly longer (P < 0.05) than oral or i.v. administration most likely due to a prolonged absorption phase. The apparent Vz of SQ ondansetron was significantly longer than oral or i.v. administration, which likely reflects delivery to a peripheral compartment. Dose interval modeling determined that twice daily administration at 0.5 mg/kg is likely inadequate to maintain serum concentrations within the therapeutic range; higher or more frequent doses may be needed. SQ administration of ondansetron to healthy cats is more bioavailable, has a longer t1/2 λ and maintains therapeutic concentrations longer than oral administration. This information may be helpful in the palliation of nausea and vomiting in patients with chronic disease.
Oral ondansetron is not as bioavailable in cats (32%) as it is in humans (62%); however, it is significantly more bioavailable in cats than in rats and dogs (<10%), where poor bioavailability is attributed to high first-pass metabolism (Saynor & Dixon, 1989; Yang & Lee, 2008). Hepatic oxidative metabolism accounts for nearly 95% of ondansetron clearance in humans and rats and <5% of the drug undergoes renal excretion (Roila & Del Favero, 1995). Ondansetron metabolites do not play a role in the activity of the drug. The drug is 53% protein bound in rats and 70% protein bound in humans, which is not perceived to affect clinical pharmacokinetics (Simpson & Hicks, 1996). Poor bioavailability should be taken into account when determining a route of administration for a patient as individual oral bioavailability ranged from 11 to 50% in the cats used in this study. In contrast, SQ bioavailability was significantly better than oral bioavailability ranging from 41 to 100%. The t1/2 λ of i.v. ondansetron appears to be shorter in cats than it is in humans even when differences in dosage regime are taken into account. In humans, 8 mg of oral or i.v. ondansetron (approximately 0.15 mg/kg) is typically prescribed, and this dose results in a t1/2 λ that ranges from 3 to 5.5 h, whereas in cats in this study, an average dose of 0.4 mg/kg resulted in a oral and i.v. t1/2 λ that ranged from of 0.8 to 2.9 h (Roila & Del Favero, 1995). The t1/2 λ of ondansetron in cats is however longer than in rats and dogs, where the t1/2 λ of 1 mg/kg ondansetron i.v. is only 10 and 30 min, respectively (Saynor & Dixon, 1989).In contrast to a t1/2 λ of 0.8–2.9 h for i.v. administration in cats, the t1/2 λ of SQ ondansetron in cats ranged from 2.6 to 3.8 h. The pharmacokinetics of subcutaneous ondansetron administration has not been assessed in humans so no comparison is possible (Simpson & Hicks, 1996).
Dose interval modeling revealed that SQ ondansetron appeared superior to other administration routes at maintaining serum concentrations within the targeted therapeutic range. But it also appears unlikely that twice daily administration would adequately maintain levels during chronic administration. A limitation of this model is that the postulated therapeutic range – extrapolated from a previously published pharmacodynamic study – may not be accurate particularly if applied to repeated administration for chronic disease states (Santos et al., 2011). Previous authors have commented that the pharmacokinetic–pharmacodynamic relationship is not entirely clear in humans (Roila & Del Favero, 1995; Simpson & Hicks, 1996). Various dosage regimes have been reported in humans to be effective for control of uremic nausea and vomiting. A placebocontrolled clinical trial will be necessary to confirm which dose would be clinically effective for palliation of chronic nausea and vomiting in cats.
This study did not assess the pharmacokinetics of ondansetron in elderly cats or those with renal or hepatic impairment. In humans, there are age-related changes in ondansetron pharmacokinetics including increased bioavailability, AUC, Cmax, and decreased CL resulting in an average increase in t1/2 λ of 56%. These changes are likely due to the effect of decreased hepatic blood flow in the elderly on the drug’s high first-pass metabolism. Despite this, dose alteration is not recommended based solely on patient age in humans (Roila & Del Favero, 1995). Due to the low percentage of renal excretion it is unlikely that drug pharmacokinetics are affected by renal disease to the extent that dose alteration is necessary (Roila & Del Favero, 1995; Simpson & Hicks, 1996), which was supported by a previous study assessing ondansetron administration in human systemic lupus erythematosis patients (Amantea et al., 1993). In humans, hepatic impairment does however result in increased bioavailability and AUC, likely due to a decrease in first-pass effect, as well as decreased clearance due to hepatic metabolism with subsequent prolongation in t1/2 λ (Figg et al., 1996). Thus, further study is needed to determine the effects of age, renal, and hepatic impairment on ondansetron pharmacokinetics in cats to better tailor dosing to individual patients.
In conclusion, ondansetron has been previously used to treat acute and chemotherapy-induced vomiting in cats (Ogilvie & Moore, 2001; Trepanier, 2010; Santos et al., 2011). A growing interest in utilization of ondansetron for palliation of nausea and vomiting in chronic disease (i.e. chronic kidney disease) and anecdotal reports of efficacy have sparked a need for additional information on pharmacokinetics in cats. Based on the results of this study, ondansetron administered subcutaneously to healthy cats is more bioavailable, has a longer t1/2 λ, and maintains therapeutic concentrations longer than oral administration and may provide owners a welcome alternative to oral administration in cats with chronic disease.
ACKNOWLEDGMENTS
This study was funded by a grant from the Angelo Fund for Feline Therapeutics and by the University of Colorado Shared Resource Grant (P30CA046934).
REFERENCES
- Amantea MA, Yarboro C, Forrest A, Pucino F, Kippel JH. Population pharmacokinetics of intravenous and oral ondansetron in patients with systemic lupus erythematosis (SLE) Journal of Clinical Pharmacology. 1993;33:1004. [Google Scholar]
- Bansal S, DeStefano A. Key elements of bioanalytical method validation for small molecules. The AAPS Journal. 2007;9:E109–E114. doi: 10.1208/aapsj0901011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figg WD, Dukes GE, Pritchard JF, Hermann DJ, Lesesne HR, Carson SW, Songer SS, Powell JR, Hak LJ. Pharmacokinetics of ondansetron in patients with hepatic insufficiency. Journal of Clinical Pharmacology. 1996;36:206–215. doi: 10.1002/j.1552-4604.1996.tb04190.x. [DOI] [PubMed] [Google Scholar]
- Ljutic D, Perkovic D, Rumboldt Z, Bagatin J, Hozo I, Pivac N. Comparison of ondansetron with metoclopramide in the symptomatic relief of uremia-induced nausea and vomiting. Kidney & Blood Pressure Research. 2002;25:61–64. doi: 10.1159/000049437. [DOI] [PubMed] [Google Scholar]
- O’Connor NR, Corcoran AM. End-stage renal disease: symptom management and advance care planning. American Family Physician. 2012;85:705–710. [PubMed] [Google Scholar]
- Ogilvie GK, Moore AS. Feline Oncology. Princeton, NJ: Veterinary Learning Systems; 2001. [Google Scholar]
- Roila F, Del Favero A. Ondansetron clinical pharmacokinetics. Clinical Pharmacokinetics. 1995;29:95–109. doi: 10.2165/00003088-199529020-00004. [DOI] [PubMed] [Google Scholar]
- Santos LC, Ludders JW, Erb HN, Martin-Flores M, Basher KL, Kirch P. A randomized, blinded, controlled trial of the antiemetic effect of ondansetron on dexmedetomidine-induced emesis in cats. Veterinary Anaesthesia and Analgesia. 2011;38:320–327. doi: 10.1111/j.1467-2995.2011.00619.x. [DOI] [PubMed] [Google Scholar]
- Saynor DA, Dixon CM. The metabolism of ondansetron. European Journal of Cancer & Clinical Oncology. 1989;25(Suppl. 1):S75–S77. [PubMed] [Google Scholar]
- Sedlacek HS, Ramsey DS, Boucher JF, Eagleson JS, Conder GA, Clemence RG. Comparative efficacy of maropitant and selected drugs in preventing emesis induced by centrally or peripherally acting emetogens in dogs. Journal of Veterinary Pharmacology and Therapeutics. 2008;31:533–537. doi: 10.1111/j.1365-2885.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- Simpson KH, Hicks FM. Clinical pharmacokinetics of ondansetron. A review. The Journal of Pharmacy and Pharmacology. 1996;48:774–781. doi: 10.1111/j.2042-7158.1996.tb03973.x. [DOI] [PubMed] [Google Scholar]
- Trepanier L. Acute vomiting in cats: rational treatment selection. Journal of Feline Medicine and Surgery. 2010;12:225–230. doi: 10.1016/j.jfms.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Park SY, Oh KH, Min Y, Lee YJ, Lee SY, Sohn UD. Characteristics of 5-hydroxytryptamine receptors involved in contraction of feline ileal longitudinal smooth muscle. The Korean Journal of Physiology & Pharmacology: Official Journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2011;15:267–272. doi: 10.4196/kjpp.2011.15.5.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Lee MG. Dose-independent pharmacokinetics of ondansetron in rats: contribution of hepatic and intestinal first-pass effects to low bioavailability. Biopharmaceutics & Drug Disposition. 2008;29:414–426. doi: 10.1002/bdd.628. [DOI] [PubMed] [Google Scholar]