FIGURE 2.
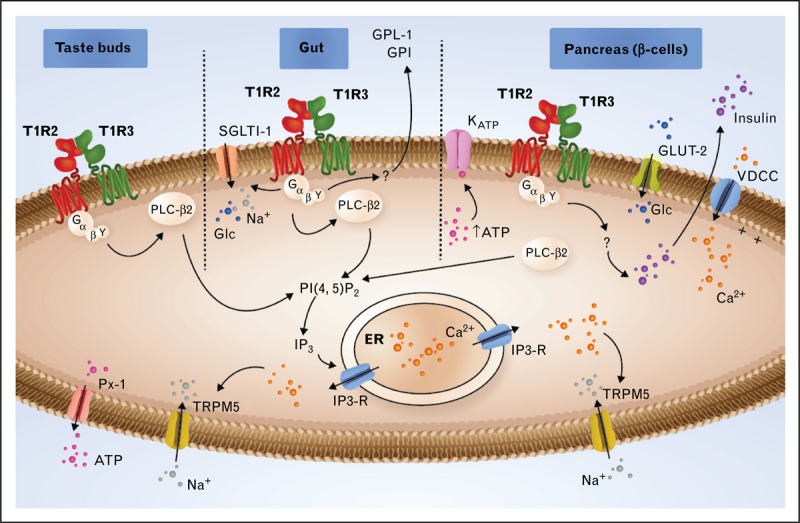
Signaling through T1R2/T1R3 in type II cells of taste buds, in the gut and in the β cells of the pancreas. In the taste buds, activated T1R2/T1R3 interacts with heterotrimeric G proteins comprising α-gustducin, Gβ3, and Gγ13. After the dissociation of the G protein subunits, the Gβγ subunit interacts with phospholipase C-β2 (PLC-β2), which in turn cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-triphosphate (IP3), producing diacylglycerol (DAG). IP3 stimulates Ca2+ release from the endoplasmic reticulum (ER) via type III IP3 receptor activation (IP3-R). The elevated intracellular Ca2+ activates the TRPM5 channel, leading to membrane depolarization, which enables the ATP channel Pannexin-1 (PX1) to open and release ATP, which stimulates efferent nerve fibers. In the gut, the signal transduction pathway is similar to that described in taste buds. The glucose (Glc) stimulation of the T1R2/T1R3 receptor triggers the secretion of two incretins, GLP-1 and GIP, and increases the expression of sodium-glucose cotransporter-1 (SGLT-1) to the plasma membrane. In the β cells of the pancreas, Glc is transported by glucose transporter-2 (GLUT-2). Glycolysis leads to an ATP increase, leading to KATP channel closure, which causes depolarization. This depolarization, in turn, activates the voltage-dependent calcium channel (VDCC), leading to the accumulation of Ca2+ in the cytoplasm and to insulin secretion. The T1R2/T1R3 receptor has been proposed to be implicated in the regulation of insulin secretion. ATP, adenosine triphosphate; GIP, glucose-dependent insulinotropic peptide; GLP-1, glucagon-like peptide-1; TRPM5, transient receptor potential cation channel subfamily M member 5.
